Synthesis of fluorapatite-hydroxyapatite nanoparticles and toxicity investigations
- PMID: 21499417
- PMCID: PMC3075893
- DOI: 10.2147/IJN.S15461
Synthesis of fluorapatite-hydroxyapatite nanoparticles and toxicity investigations
Abstract
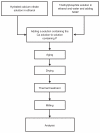
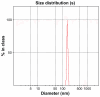
In this study, calcium phosphate nanoparticles with two phases, fluorapatite (FA; Ca(10)(PO(4))(6)F(2)) and hydroxyapatite (HA; Ca(10)(PO(4))(6)(OH)(2)), were prepared using the solgel method. Ethyl phosphate, hydrated calcium nitrate, and ammonium fluoride were used, respectively, as P, Ca, and F precursors with a Ca:P ratio of 1:72. Powders obtained from the sol-gel process were studied after they were dried at 80°C and heat treated at 550°C. The degree of crystallinity, particle and crystallite size, powder morphology, chemical structure, and phase analysis were investigated by scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and Zetasizer experiments. The results of XRD analysis and FTIR showed the presence of hydroxyapatite and fluorapatite phases. The sizes of the crystallites estimated from XRD patterns using the Scherrer equation and the crystallinity of the hydroxyapatite phase were about 20 nm and 70%, respectively. Transmission electron microscope and SEM images and Zetasizer experiments showed an average size of 100 nm. The in vitro behavior of powder was investigated with mouse fibroblast cells. The results of these experiments indicated that the powders were biocompatible and would not cause toxic reactions. These compounds could be applied for hard-tissue engineering.
Keywords: biocompatibility; fluorapatite; hydroxyapatite; nanoparticles; sol-gel.
Figures





Similar articles
-
Synthesis of high purity hydroxyapatite nanopowder via sol-gel combustion process.J Mater Sci Mater Med. 2009 Jun;20(6):1223-7. doi: 10.1007/s10856-008-3685-x. Epub 2009 Jan 10. J Mater Sci Mater Med. 2009. PMID: 19132503
-
Facile synthesis of both needle-like and spherical hydroxyapatite nanoparticles: effect of synthetic temperature and calcination on morphology, crystallite size and crystallinity.Mater Sci Eng C Mater Biol Appl. 2014 Sep;42:83-90. doi: 10.1016/j.msec.2014.05.032. Epub 2014 May 23. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 25063096
-
One- and three-dimensional growth of hydroxyapatite nanowires during sol-gel-hydrothermal synthesis.ACS Appl Mater Interfaces. 2012 Mar;4(3):1490-9. doi: 10.1021/am201735k. Epub 2012 Feb 15. ACS Appl Mater Interfaces. 2012. PMID: 22296410
-
Effect of aging temperature on formation of sol-gel derived fluor-hydroxyapatite nanoparticles.J Nanosci Nanotechnol. 2010 Apr;10(4):2892-6. doi: 10.1166/jnn.2010.1397. J Nanosci Nanotechnol. 2010. PMID: 20355519
-
Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol-gel method. Optimisation, characterisation and rheology.Dent Mater. 2013 Feb;29(2):166-73. doi: 10.1016/j.dental.2012.11.008. Epub 2012 Dec 4. Dent Mater. 2013. PMID: 23218445
Cited by
-
Acidic Microenvironment-Sensitive Core-Shell Microcubes: The Self-assembled and the Therapeutic Effects for Caries Prevention.Eur J Dent. 2023 Jul;17(3):863-870. doi: 10.1055/s-0042-1757464. Epub 2022 Dec 19. Eur J Dent. 2023. PMID: 36535661 Free PMC article.
-
Effect of Fluorohydroxyapatite on Biological and Physical Properties of MTA Angelus.ScientificWorldJournal. 2023 Nov 6;2023:7532898. doi: 10.1155/2023/7532898. eCollection 2023. ScientificWorldJournal. 2023. PMID: 37964892 Free PMC article.
-
A Highly Ordered, Nanostructured Fluorinated CaP-Coated Melt Electrowritten Scaffold for Periodontal Tissue Regeneration.Adv Healthc Mater. 2021 Nov;10(21):e2101152. doi: 10.1002/adhm.202101152. Epub 2021 Aug 3. Adv Healthc Mater. 2021. PMID: 34342173 Free PMC article.
-
Comparative study of pulpal response following direct pulp capping using synthesized fluorapatite and hydroxyapatite nanoparticles.BMC Oral Health. 2025 Jan 4;25(1):17. doi: 10.1186/s12903-024-05285-4. BMC Oral Health. 2025. PMID: 39754111 Free PMC article.
-
Culture & differentiation of mesenchymal stem cell into osteoblast on degradable biomedical composite scaffold: In vitro study.Indian J Med Res. 2015 Dec;142(6):747-58. doi: 10.4103/0971-5916.174568. Indian J Med Res. 2015. PMID: 26831424 Free PMC article.
References
-
- Cengiz B, Gokce Y, Yildiz N, et al. Synthesis and characterization of hydroxyapatite nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2008;322:1–3. 29–33.
-
- Larsen MJ, Jensen SJ. Solubility, unit cell dimensions and crystallinity of fluoridated human dental enamel. Arch Oral Biol. 1989;34(12):969–973. - PubMed
-
- Finkelstein MJ, Nancollas GH. Trace fluoride and its role in enamel mineralization. J Biomed Mater Res. 1980;14(4):533–535. - PubMed
-
- Legeros RZ, Silverstone LM, Daculsi G, Kerebel LM. In vitro carieslike lesion formation F-containing tooth enamel. J Dent Res. 1985;62:138–144. - PubMed
-
- Shackelford J. Bioceramics (Advanced Ceramics) New Jersey, USA: Prentice Hall; 1992.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

