Preparation, characterization and targeting of micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles to cancer cells
- PMID: 21499429
- PMCID: PMC3075905
- DOI: 10.2147/IJN.S16144
Preparation, characterization and targeting of micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles to cancer cells
Abstract
Background: The purpose of this study was to develop a method for targeted delivery of 10-hydroxycamptothecin (HCPT)-loaded nanoparticles (NPs) to cancer cells.
Methods: We first used a supercritical antisolvent process to prepare micronized HCPT (nHCPT), and then folate-conjugated human serum albumin (HSA) nHCPT-loaded NPs (FA-HSA-nHCPT-NPs) were prepared using a NP-coated method combined with a desolvation technique. The amount of folate conjugation was 16 μg · mg(-1) HSA.
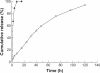
Results: The particle size of the spherical nHCPT microparticles obtained was 118.5 ± 6.6 nm. The particle size and zeta potential of the FA-HSA-nHCPT-NPs were 233.9 ± 1.2 nm and -25.23 ± 2.98 mV, respectively. The FA-HSA-nHCPT-NPs exhibited a smooth surface and a distinct spherical shape, and the results of differential scanning calorimetry and X-ray diffraction indicated that the FA-HSA-nHCPT-NPs presented in a nanostructured amorphous state. The FA-HSA-nHCPT-NPs showed sustained-release characteristics for 120 hours in vitro, with a drug-loading content of 7.3% and an encapsulating efficiency of 79.1%.
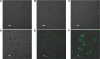
Conclusion: The FA-NPs were effective delivery systems for uptake by SGC7901 cells compared with folate-free NPs. These results suggest that a NP-coated method combined with a desolvation technique is effective for preparing NPs with drugs having poor solubility in water and most organic solvents, using albumin as the wall material. FA-HSA-NPs are a stable delivery system and have the potential for targeted delivery of anticancer drugs.
Keywords: 10-hydroxycamptothecin; desolvation technique; folate; human serum albumin; nanoparticle-coated; targeted delivery.
Figures




Similar articles
-
Preparation of 10-hydroxycamptothecin-loaded glycyrrhizic acid-conjugated bovine serum albumin nanoparticles for hepatocellular carcinoma-targeted drug delivery.Int J Nanomedicine. 2013;8:1207-22. doi: 10.2147/IJN.S40493. Epub 2013 Mar 27. Int J Nanomedicine. 2013. PMID: 23569373 Free PMC article.
-
Pharmacokinetics and Tissue Distribution of Folate-Decorated Human Serum Albumin Loaded With Nano-Hydroxycamptothecin for Tumor Targeting.J Pharm Sci. 2016 Jun;105(6):1874-1880. doi: 10.1016/j.xphs.2016.03.016. Epub 2016 Apr 26. J Pharm Sci. 2016. PMID: 27129905
-
Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles.Int J Nanomedicine. 2010 Sep 20;5:669-77. doi: 10.2147/ijn.s12918. Int J Nanomedicine. 2010. PMID: 20957218 Free PMC article.
-
Folate-engineered chitosan nanoparticles: next-generation anticancer nanocarriers.Mol Cancer. 2024 Oct 31;23(1):244. doi: 10.1186/s12943-024-02163-z. Mol Cancer. 2024. PMID: 39482651 Free PMC article. Review.
-
Transferrin-coated gadolinium-labeled human serum albumin nanoparticles.2013 Apr 30 [updated 2013 May 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2013 Apr 30 [updated 2013 May 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23700643 Free Books & Documents. Review.
Cited by
-
Process optimization for the preparation of oligomycin-loaded folate-conjugated chitosan nanoparticles as a tumor-targeted drug delivery system using a two-level factorial design method.Int J Nanomedicine. 2011;6:3429-41. doi: 10.2147/IJN.S27157. Epub 2011 Dec 20. Int J Nanomedicine. 2011. PMID: 22267927 Free PMC article.
-
Carrier-Free, Dual-Functional Nanorods Via Self-Assembly Of Pure Drug Molecules For Synergistic Chemo-Photodynamic Therapy.Int J Nanomedicine. 2019 Nov 5;14:8665-8683. doi: 10.2147/IJN.S224704. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31806963 Free PMC article.
-
Therapeutic potential of human serum albumin nanoparticles encapsulated actinonin in murine model of lung adenocarcinoma.Drug Deliv. 2022 Dec;29(1):2403-2413. doi: 10.1080/10717544.2022.2067600. Drug Deliv. 2022. PMID: 35892161 Free PMC article.
-
Preparation of 10-hydroxycamptothecin-loaded glycyrrhizic acid-conjugated bovine serum albumin nanoparticles for hepatocellular carcinoma-targeted drug delivery.Int J Nanomedicine. 2013;8:1207-22. doi: 10.2147/IJN.S40493. Epub 2013 Mar 27. Int J Nanomedicine. 2013. PMID: 23569373 Free PMC article.
-
Dasatinib-loaded albumin nanoparticles possess diminished endothelial cell barrier disruption and retain potent anti-leukemia cell activity.Oncotarget. 2016 Aug 2;7(31):49699-49709. doi: 10.18632/oncotarget.10435. Oncotarget. 2016. PMID: 27391073 Free PMC article.
References
-
- Wang J, Wang R, Li LB. Preparation and properties of hydroxycamptothecin-loaded nanoparticles made of amphiphilic copolymer and normal polymer. J Colloid Interface Sci. 2009;336(2):808–813. - PubMed
-
- Pu X, Sun J, Wang Y, et al. Development of a chemically stable 10-hydroxycamptothecin nanosuspension. Int J Pharm. 2009;379(1):167–173. - PubMed
-
- Ertl B, Platzer P, Wirth M, Gabor F. Poly (D,L-lactic-co-glycolic acid) microspheres for sustained delivery and stabilization of camptothecin. J Control Release. 1999;61(3):305–317. - PubMed
-
- Shenderova A, Burke TG, Schwendeman SP. Stabilization of 10-hydroxycamptothecin in poly (lactide-co-glycolide) microsphere delivery vehicles. Pharm Res. 1997;14(10):1406–1414. - PubMed
-
- O’Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34(10):1500–1508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

