Structural, Mechanistic and Coordination Chemistry of Relevance to the Biosynthesis of Iron-Sulfur and Related Iron Cofactors
- PMID: 21499539
- PMCID: PMC3074115
- DOI: 10.1016/j.ccr.2010.10.016
Structural, Mechanistic and Coordination Chemistry of Relevance to the Biosynthesis of Iron-Sulfur and Related Iron Cofactors
Abstract
Iron-sulfur clusters are an important class of protein-bound prosthetic center that find wide utility in nature. Roles include electron transfer, enzyme catalysis, protein structure stabilization, and regulation of gene expression as transcriptional and translational sensors. In eukaryotes their biosynthesis requires a complex molecular machinery that is located within the mitochondrion, while bacteria exhibit up to three independent cluster assembly pathways. All of these paths share common themes. This review summarizes some key structural and functional properties of three central proteins dedicated to the Fe-S cluster assembly process: namely, the sulfide donor (cysteine desulfurase); iron donor (frataxin), and the iron-sulfur cluster scaffold protein (IscU/ISU).
Figures


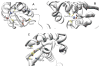
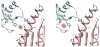





References
-
- Peters JW, Stowell MH, Soltis SM, Finnegan MG, Johnson MK, Rees DC. Biochemistry. 1997;36:1181–1187. - PubMed
-
- Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247–281. - PubMed
-
- Volbeda A, Charon MH, Piras C, Hatchikian EC, Frey M, Fontecilla-Camps JC. Nature. 1995;373:580–587. - PubMed
-
- Iwata S, Saynovits M, Link TA, Michel H. Structure. 1996;4:567–579. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
