Characterization of silvestrol pharmacokinetics in mice using liquid chromatography-tandem mass spectrometry
- PMID: 21499689
- PMCID: PMC3160157
- DOI: 10.1208/s12248-011-9273-x
Characterization of silvestrol pharmacokinetics in mice using liquid chromatography-tandem mass spectrometry
Abstract
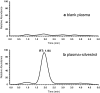
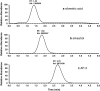
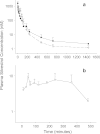
A sensitive and specific liquid chromatography-tandem mass spectrometry method was developed and validated for the quantification of the plant natural product silvestrol in mice, using ansamitocin P-3 as the internal standard. The method was validated in plasma with a lower limit of quantification of 1 ng/mL, accuracy ranging from 87 to 114%, and precision (coefficient of variation) below 15%. The validated method was used to characterize pharmacokinetics in C57BL/6 mice and metabolism in mouse, human and rat plasma, and liver microsomes. Mice were dosed with silvestrol formulated in hydroxypropyl-β-cyclodextrin via intravenous, intraperitoneal, and oral routes followed by blood sampling up to 24 h. Intraperitoneal systemic availability was 100%, but oral administration resulted in only 1.7% bioavailability. Gradual degradation of silvestrol was observed in mouse and human plasma, with approximately 60% of the parent drug remaining after 6 h. In rat plasma, however, silvestrol was completely converted to silvestric acid (SA) within 10 min. Evaluation in microsomes provided further evidence that the main metabolite formed was SA, which subsequently showed no cytotoxic or cytostatic activity in a silvestrol-sensitive lymphoblastic cell line. The ability of the analytical assay to measure tissue levels of silvestrol was evaluated in liver, brain, kidney, and spleen. Results indicated the method was capable of accurately measuring tissue levels of silvestrol and suggested it has a relatively low distribution to brain. Together, these data suggest an overall favorable pharmacokinetic profile of silvestrol in mice and provide crucial information for its continued development toward potential clinical testing.
Figures




Similar articles
-
Plasma pharmacokinetics and bioavailability of verticillin A following different routes of administration in mice using liquid chromatography tandem mass spectrometry.J Pharm Biomed Anal. 2017 May 30;139:187-192. doi: 10.1016/j.jpba.2017.02.051. Epub 2017 Mar 1. J Pharm Biomed Anal. 2017. PMID: 28284083 Free PMC article.
-
Analysis of bacopaside I in biomatrices using liquid chromatography-tandem mass spectrometry: Pharmacokinetics and brain distribution in Swiss-albino mice.J Pharm Biomed Anal. 2016 Jun 5;125:101-9. doi: 10.1016/j.jpba.2016.03.002. Epub 2016 Mar 3. J Pharm Biomed Anal. 2016. PMID: 27017568
-
Plasma pharmacokinetics and tissue distribution study of cajaninstilbene acid in rats by liquid chromatography with tandem mass spectrometry.J Pharm Biomed Anal. 2010 Jun 5;52(2):273-9. doi: 10.1016/j.jpba.2010.01.004. Epub 2010 Jan 15. J Pharm Biomed Anal. 2010. PMID: 20116956
-
Determination and pharmacokinetic study of chiisanogenin in rat plasma by ultra performance liquid chromatography-tandem mass spectrometry.Phytochem Anal. 2011 May-Jun;22(3):225-9. doi: 10.1002/pca.1269. Epub 2010 Nov 2. Phytochem Anal. 2011. PMID: 21046686
-
An LC-MS/MS method for quantification of demethylzeylasteral, a novel immunosuppressive agent in rat plasma and the application to a pharmacokinetic study.Biomed Chromatogr. 2018 Aug;32(8):e4247. doi: 10.1002/bmc.4247. Epub 2018 Apr 23. Biomed Chromatogr. 2018. PMID: 29574824
Cited by
-
Discovery of Anticancer Agents of Diverse Natural Origin.Anticancer Res. 2016 Nov;36(11):5623-5637. doi: 10.21873/anticanres.11146. Anticancer Res. 2016. PMID: 27793884 Free PMC article. Review.
-
Repurposing host-based therapeutics to control coronavirus and influenza virus.Drug Discov Today. 2019 Mar;24(3):726-736. doi: 10.1016/j.drudis.2019.01.018. Epub 2019 Jan 31. Drug Discov Today. 2019. PMID: 30711575 Free PMC article. Review.
-
The translation inhibitor silvestrol exhibits direct anti-tumor activity while preserving innate and adaptive immunity against EBV-driven lymphoproliferative disease.Oncotarget. 2015 Feb 20;6(5):2693-708. doi: 10.18632/oncotarget.2098. Oncotarget. 2015. PMID: 25393910 Free PMC article.
-
Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses.Antiviral Res. 2018 Feb;150:123-129. doi: 10.1016/j.antiviral.2017.12.010. Epub 2017 Dec 16. Antiviral Res. 2018. PMID: 29258862 Free PMC article.
-
eIF4F-mediated dysregulation of mRNA translation in cancer.RNA. 2025 Feb 19;31(3):416-428. doi: 10.1261/rna.080340.124. RNA. 2025. PMID: 39809544 Free PMC article. Review.
References
-
- King ML. X-Ray crystal-structure of rocaglamide, a novel antileukemic 1H-cyclopenta[b]benzofuran from Aglaia elliptifolia. J Chem Soc Chem Commun. 1982;20:1150–1151. doi: 10.1039/c39820001150. - DOI
-
- Trost BM, et al. An unusual oxidative cyclization—A synthesis and absolute stereochemical assignment of (−)-rocaglamide. J Am Chem Soc. 1990;112(24):9022–9024. doi: 10.1021/ja00180a081. - DOI
-
- Proksch P, et al. Chemistry and biological activity of rocaglamide derivatives and related compounds in Aglaia species (Meliaceae) Curr Org Chem. 2001;5(9):923–938. doi: 10.2174/1385272013375049. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

