Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection
- PMID: 21499735
- PMCID: PMC3171633
- DOI: 10.1007/s00109-011-0756-0
Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection
Abstract
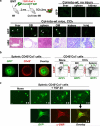
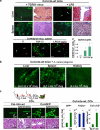
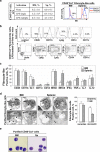
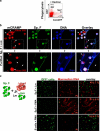
Bone marrow (BM)-derived fibrocytes are a population of CD45(+) and collagen Type I-expressing cells that migrate to the spleen and to target injured organs, such as skin, lungs, kidneys, and liver. While CD45(+)Col(+) fibrocytes contribute to collagen deposition at the site of injury, the role of CD45(+)Col(+) cells in spleen has not been elucidated. Here, we demonstrate that hepatotoxic injury (CCl(4)), TGF-β1, lipopolysaccharide, or infection with Listeria monocytogenes induce rapid recruitment of CD45(+)Col(+) fibrocyte-like cells to the spleen. These cells have a gene expression pattern that includes antimicrobial factors (myleoperoxidase, cathelicidin, and defensins) and MHC II at higher levels than found on quiescent or activated macrophages. The immune functions of these splenic CD45(+)Col(+) fibrocyte-like cells include entrapment of bacteria into extracellular DNA-based structures containing cathelicidin and presentation of antigens to naïve CD8(+) T cells to induce their proliferation. Stimulation of these splenic fibrocyte-like cells with granulocyte macrophage-colony stimulating factor or macrophage-colony stimulating factor induces downregulation of collagen expression and terminal differentiation into the dendritic cells or macrophage. Thus, splenic CD45(+)Col(+) cells are a population of rapidly mobilized BM-derived fibrocyte-like cells that respond to inflammation or infection to participate in innate and adaptive immune responses.
Figures




References
-
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi:10.1016/j.biocel.2003.10.005. - PubMed
-
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol. 2006;18:614–617. doi:10.1097/01.bor.0000245725.94887.8d. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

