Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial
- PMID: 21499862
- PMCID: PMC3548408
- DOI: 10.1245/s10434-011-1630-6
Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial
Abstract
Background: The impact of the addition of gemcitabine to 5-fluorouracil (5-FU) chemoradiation (CRT) on 5-year overall survival (OS) in resected pancreatic adenocarcinoma are presented with updated results of a phase III trial.
Methods: After resection of pancreatic adenocarcinoma, patients were randomized to pre- and post-CRT 5-FU versus pre- and post-CRT gemcitabine. 5-FU was provided continuously at 250 mg/m(2)/day, and gemcitabine was provided at 1000 mg/m(2) weekly. Both were provided over 3 weeks before and 12 weeks after CRT. CRT was provided at 50.4 Gy with continuously provided 5-FU. The primary end point was survival for all patients and for patients with tumor of the pancreatic head.
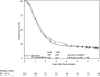
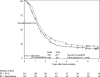
Results: Four hundred fifty-one patients were eligible. Univariate analysis showed no difference in OS. Pancreatic head tumor patients (n = 388) had a median survival and 5-year OS of 20.5 months and 22% with gemcitabine versus 17.1 months and 18% with 5-FU. On multivariate analysis, patients on the gemcitabine arm with pancreatic head tumors experienced a trend toward improved OS (P = 0.08). First site of relapse local recurrence in 28% of patients versus distant relapse in 73%.
Conclusions: The sequencing of 5-FU CRT with gemcitabine as done in this trial is not associated with a statistically significant improvement in OS. Despite local recurrence being approximately half of that reported in previous adjuvant trials, distant disease relapse still occurs in ≥ 70% of patients. These findings serve as the basis for the recently activated EORTC/U.S. Intergroup RTOG 0848 phase III adjuvant trial evaluating the impact of CRT after completion of a full course of gemcitabine.
Figures
Comment in
-
Adjuvant therapy for pancreatic cancer: a logical strategy in search of progress.Ann Surg Oncol. 2011 May;18(5):1224-8. doi: 10.1245/s10434-011-1633-3. Ann Surg Oncol. 2011. PMID: 21384245 No abstract available.
References
-
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant Chemotherapy with Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer: A Randomized Trial. JAMA. 2007;297(3):267–77. - PubMed
-
- Neuhaus P, Riess H, Post S, et al. CONKO-001: Final Results of the Randomized, Prospective, Multicenter Phase III Trial of Adjuvant Chemotherapy with Gemcitabine versus Observation in Patients with Resected Pancreatic Cancer (PC). J Clin Oncol. 2008;26(suppl LBA4504)
-
- Neoptolemos J, Buchler M, Stocken DD, et al. ESPAC-3(v2): A multicenter, international, open-label, randomized, controlled phase III trial of adjuvant 5-fluorouracil/folonic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009;27(suppl18)
-
- Regine WF, Winter K, Abrams RA, et al. Fluorouracil vs. Gemcitabine Chemotherapy Before and After Fluorouracil- based Chemoradiation Following resection of Pancreatic Adenocarcinoma: A Randomized Controlled Trial. JAMA. 2008;299(9):1019–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

