An evaluation of the emerging vaccines and immunotherapy against staphylococcal pneumonia in children
- PMID: 21501445
- PMCID: PMC3239838
- DOI: 10.1186/1471-2458-11-S3-S27
An evaluation of the emerging vaccines and immunotherapy against staphylococcal pneumonia in children
Abstract
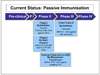
Background: Staphylococcus aureus is a commensal of human skin and nares. It is also one of the leading nosocomial pathogens in both developed and developing countries and is responsible for a wide range of life threatening infections, especially in patients who are immunocompromised, post-surgery, undergoing haemodialysis and those who are treated with catheters and ventilators. Over the past two decades, the incidence of nosocomial staphylococcal infections has increased dramatically. Currently there are at least seven vaccine and immunotherapy candidates against S. aureus in the developmental phase targeting both active and passive immunization.
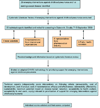
Methods: We used a modified CHNRI methodology for setting priorities in health research investments. This was done in two stages. In Stage I, we systematically reviewed the literature related to emerging vaccines against Staphylococcus aureus relevant to several criteria of interest: answerability; cost of development, production and implementation; efficacy and effectiveness; deliverability, affordability and sustainability; maximum potential impact on disease burden reduction; acceptability to the end users and health workers; and effect on equity. In Stage II, we conducted an expert opinion exercise by inviting 20 experts (leading basic scientists, international public health researchers, international policy makers and representatives of pharmaceutical companies) to participate. The policy makers and industry representatives accepted our invitation on the condition of anonymity, due to sensitive nature of their involvement in such exercises. They answered questions from CHNRI framework and their "collective optimism" towards each criterion was documented on a scale from 0 to 100%.
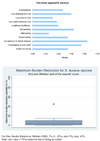
Results: The panel of experts expressed low levels of optimism (score around or below 50%) on the criteria of answerability, efficacy, maximum disease burden reduction potential, low cost of production, low cost of implementation and affordability; moderate levels of optimism (scores around 60 to 80%) that these vaccines could be developed at a low cost, and thus on the deliverability, sustainability and impact on equity; and high levels of optimism (scores above 80%) regarding acceptable of such a product to both the end-users and health workers. While assessing the candidates for passive immunization against S.aureus, the experts were poorly optimistic regarding low production cost, low implementation cost, efficacy, deliverability, sustainability, affordability and equity; moderately optimistic regarding answerability and acceptability to health workers and end-users. They were of the opinion that these interventions would have only a modest impact (3 to 5%) on the burden of childhood pneumonia. .
Conclusion: In order to provide an effective vaccine against S. aureus, a number of unresolved issues in vaccine development relating to optimal antigenic target identification, criteria for acceptable efficacy, identification of target population, commercial development limitations, optimal timing of immunization strategy, storage, cold chain requirements and cost need to be addressed properly. There is still a great deal unknown about the complex interaction between S. aureus and the human host. However, given the nature of S. aureus and the lessons learned from the recent failure of two emerging vaccines, it is clear that a multi-component vaccine is essential. Combating only one virulence factor is not sufficient in the human host but finding the right combination of factors will be very challenging.
Figures


Similar articles
-
An evaluation of the emerging vaccines against influenza in children.BMC Public Health. 2013;13 Suppl 3(Suppl 3):S14. doi: 10.1186/1471-2458-13-S3-S14. Epub 2013 Sep 17. BMC Public Health. 2013. PMID: 24564565 Free PMC article. Review.
-
An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children.BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S30. doi: 10.1186/1471-2458-11-S3-S30. BMC Public Health. 2011. PMID: 21501449 Free PMC article. Review.
-
An evaluation of respiratory administration of measles vaccine for prevention of acute lower respiratory infections in children.BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S31. doi: 10.1186/1471-2458-11-S3-S31. BMC Public Health. 2011. PMID: 21501450 Free PMC article. Review.
-
An evaluation of emerging vaccines for childhood pneumococcal pneumonia.BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S26. doi: 10.1186/1471-2458-11-S3-S26. BMC Public Health. 2011. PMID: 21501444 Free PMC article. Review.
-
An evaluation of emerging vaccines for childhood meningococcal disease.BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S29. doi: 10.1186/1471-2458-11-S3-S29. BMC Public Health. 2011. PMID: 21501447 Free PMC article. Review.
Cited by
-
Protective effect of recombinant staphylococcal enterotoxin A entrapped in polylactic-co-glycolic acid microspheres against Staphylococcus aureus infection.Vet Res. 2012 Mar 19;43(1):20. doi: 10.1186/1297-9716-43-20. Vet Res. 2012. PMID: 22429499 Free PMC article.
-
Edible Vaccines: Promises and Challenges.Mol Biotechnol. 2020 Feb;62(2):79-90. doi: 10.1007/s12033-019-00222-1. Mol Biotechnol. 2020. PMID: 31758488 Free PMC article. Review.
-
An evaluation of the emerging vaccines against influenza in children.BMC Public Health. 2013;13 Suppl 3(Suppl 3):S14. doi: 10.1186/1471-2458-13-S3-S14. Epub 2013 Sep 17. BMC Public Health. 2013. PMID: 24564565 Free PMC article. Review.
-
The Candidate Antigens to Achieving an Effective Vaccine against Staphylococcus aureus.Vaccines (Basel). 2022 Jan 27;10(2):199. doi: 10.3390/vaccines10020199. Vaccines (Basel). 2022. PMID: 35214658 Free PMC article. Review.
-
Setting priorities for development of emerging interventions against childhood pneumonia, meningitis and influenza.J Glob Health. 2012 Jun;2(1):010304. doi: 10.7189/jogh.02.010304. J Glob Health. 2012. PMID: 23198129 Free PMC article. No abstract available.
References
-
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

