An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children
- PMID: 21501449
- PMCID: PMC3231904
- DOI: 10.1186/1471-2458-11-S3-S30
An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children
Abstract
Background: Respiratory Syncytial Virus (RSV) is the leading cause of acute lower respiratory infections (ALRI) in children. It is estimated to cause approximately 33.8 million new episodes of ALRI in children annually, 96% of these occurring in developing countries. It is also estimated to result in about 53,000 to 199,000 deaths annually in young children. Currently there are several vaccine and immunoprophylaxis candidates against RSV in the developmental phase targeting active and passive immunization.
Methods: We used a modified CHNRI methodology for setting priorities in health research investments. This was done in two stages. In Stage I, we systematically reviewed the literature related to emerging vaccines against RSV relevant to 12 criteria of interest. In Stage II, we conducted an expert opinion exercise by inviting 20 experts (leading basic scientists, international public health researchers, international policy makers and representatives of pharmaceutical companies). The policy makers and industry representatives accepted our invitation on the condition of anonymity, due to the sensitive nature of their involvement in such exercises. They answered questions from the CHNRI framework and their "collective optimism" towards each criterion was documented on a scale from 0 to 100%.
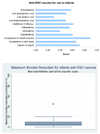
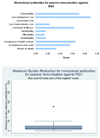
Results: In the case of candidate vaccines for active immunization of infants against RSV, the experts expressed very low levels of optimism for low product cost, affordability and low cost of development; moderate levels of optimism regarding the criteria of answerability, likelihood of efficacy, deliverability, sustainability and acceptance to end users for the interventions; and high levels of optimism regarding impact on equity and acceptance to health workers. While considering the candidate vaccines targeting pregnant women, the panel expressed low levels of optimism for low product cost, affordability, answerability and low development cost; moderate levels of optimism for likelihood of efficacy, deliverability, sustainability and impact on equity; high levels of optimism regarding acceptance to end users and health workers. The group also evaluated immunoprophylaxis against RSV using monoclonal antibodies and expressed no optimism towards low product cost; very low levels of optimism regarding deliverability, affordability, sustainability, low implementation cost and impact on equity; moderate levels of optimism against the criteria of answerability, likelihood of efficacy, acceptance to end-users and health workers; and high levels of optimism regarding low development cost. They felt that either of these vaccines would have a high impact on reducing burden of childhood ALRI due to RSV and reduce the overall childhood ALRI burden by a maximum of about 10%.
Conclusion: Although monoclonal antibodies have proven to be effective in providing protection to high-risk infants, their introduction in resource poor settings might be limited by high cost associated with them. Candidate vaccines for active immunization of infants against RSV hold greatest promise. Introduction of a low cost vaccine against RSV would reduce the inequitable distribution of burden due to childhood ALRI and will most likely have a high impact on morbidity and mortality due to severe ALRI.
Figures





References
-
- Nair H, Nokes D, Gessner B, Dherani M, Madhi S, Singleton R, O'Brien K, Roca A, Wright P, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih E, Ngama M, Munywoki P, Kartasasmita C, Simoes E, Rudan I, Weber M, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Stang PE. The Economic Burden of Respiratory Syncytial Virus-Associated Bronchiolitis Hospitalizations. Arch Pediatr Adolesc Med. 2001;155(1):95–96. - PubMed
-
- Wright PF, Cutts FT. Generic protocol to examine the incidence of lower respiratory infection due to respiratory syncytial virus in children less than 5 years of age. Geneva: World Health Organization; 2000. p. 34.
-
- Singleton RJ, Redding GJ, Lewis TC, Martinez P, Bulkow L, Morray B, Peters H, Gove J, Jones C, Stamey D, Talkington DF, DeMain J, Bernert JT, Butler JC. Sequelae of Severe Respiratory Syncytial Virus Infection in Infancy and Early Childhood Among Alaska Native Children. Pediatrics. 2003;112:285–290. doi: 10.1542/peds.112.2.285. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

