Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo
- PMID: 21501498
- PMCID: PMC3090367
- DOI: 10.1186/1471-2407-11-144
Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo
Abstract
Background: Medulloblastoma is the most common brain tumor in children, and its prognosis is worse than for many other common pediatric cancers. Survivors undergoing treatment suffer from serious therapy-related side effects. Thus, it is imperative to identify safer, effective treatments for medulloblastoma. In this study we evaluated the anti-cancer potential of curcumin in medulloblastoma by testing its ability to induce apoptosis and inhibit tumor growth in vitro and in vivo using established medulloblastoma models.
Methods: Using cultured medulloblastoma cells, tumor xenografts, and the Smo/Smo transgenic medulloblastoma mouse model, the antitumor effects of curcumin were tested in vitro and in vivo.
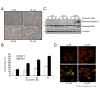
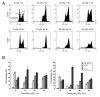
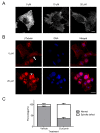
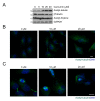
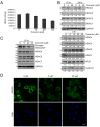
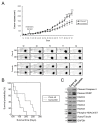
Results: Curcumin induced apoptosis and cell cycle arrest at the G2/M phase in medulloblastoma cells. These effects were accompanied by reduced histone deacetylase (HDAC) 4 expression and activity and increased tubulin acetylation, ultimately leading to mitotic catastrophe. In in vivo medulloblastoma xenografts, curcumin reduced tumor growth and significantly increased survival in the Smo/Smo transgenic medulloblastoma mouse model.
Conclusions: The in vitro and in vivo data suggest that curcumin has the potential to be developed as a therapeutic agent for medulloblastoma.
Figures
References
-
- Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y. et al.Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14(2):123–134. doi: 10.1016/j.ccr.2008.07.005. - DOI - PMC - PubMed
-
- Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL. et al.Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

