Mechanism of Mss116 ATPase reveals functional diversity of DEAD-Box proteins
- PMID: 21501623
- PMCID: PMC3125984
- DOI: 10.1016/j.jmb.2011.04.004
Mechanism of Mss116 ATPase reveals functional diversity of DEAD-Box proteins
Abstract
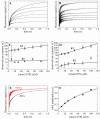
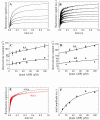
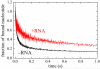
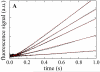
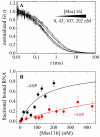
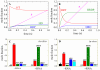
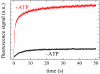
Mss116 is a Saccharomyces cerevisiae mitochondrial DEAD-box RNA helicase protein that is essential for efficient in vivo splicing of all group I and group II introns and for activation of mRNA translation. Catalysis of intron splicing by Mss116 is coupled to its ATPase activity. Knowledge of the kinetic pathway(s) and biochemical intermediates populated during RNA-stimulated Mss116 ATPase is fundamental for defining how Mss116 ATP utilization is linked to in vivo function. We therefore measured the rate and equilibrium constants underlying Mss116 ATP utilization and nucleotide-linked RNA binding. RNA accelerates the Mss116 steady-state ATPase ∼7-fold by promoting rate-limiting ATP hydrolysis such that inorganic phosphate (P(i)) release becomes (partially) rate-limiting. RNA binding displays strong thermodynamic coupling to the chemical states of the Mss116-bound nucleotide such that Mss116 with bound ADP-P(i) binds RNA more strongly than Mss116 with bound ADP or in the absence of nucleotide. The predominant biochemical intermediate populated during in vivo steady-state cycling is the strong RNA-binding Mss116-ADP-P(i) state. Strong RNA binding allows Mss116 to fulfill its biological role in the stabilization of group II intron folding intermediates. ATPase cycling allows for transient population of the weak RNA-binding ADP state of Mss116 and linked dissociation from RNA, which is required for the final stages of intron folding. In cases where Mss116 functions as a helicase, the data collectively favor a model in which ATP hydrolysis promotes a weak-to-strong RNA binding transition that disrupts stable RNA duplexes. The subsequent strong-to-weak RNA binding transition associated with P(i) release dissociates Mss116-RNA complexes, regenerating free Mss116.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



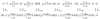
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

