Cortical feedback regulation of input to visual cortex: role of intrageniculate interneurons
- PMID: 21502287
- PMCID: PMC3139080
- DOI: 10.1113/jphysiol.2011.205542
Cortical feedback regulation of input to visual cortex: role of intrageniculate interneurons
Abstract
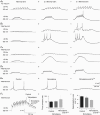
Neurons in the dorsal lateral geniculate nucleus (dLGN) process and transmit visual signals from retina to visual cortex. The processing is dynamically regulated by cortical excitatory feedback to neurons in dLGN, and synaptic short-term plasticity (STP) has an important role in this regulation. It is known that corticogeniculate synapses on thalamocortical (TC) projection-neurons are facilitating, but type and characteristics of STP of synapses on inhibitory interneurons in dLGN are unknown. We studied STP at corticogeniculate synapses on interneurons and compared the results with STP-characteristics of corticogeniculate synapses on TC neurons to gain insights into the dynamics of cortical regulation of processing in dLGN. We studied neurons in thalamic slices from glutamate decarboxylase 67 (GAD67)–green fluorescent protein (GFP) knock-in mice and made whole-cell recordings of responses evoked by electrical paired-pulse and pulse train stimulation of cortical afferents. We found that cortical excitations of interneurons and TC neurons have distinctly different properties. A single pulse evoked larger EPSCs in interneurons than in TC neurons. However, repetitive stimulation induced frequency-dependent depression of interneurons in contrast to the facilitation of TC neurons. Thus, through these differences of STP mechanisms, the balance of cortical excitation of the two types of neurons could change during stimulation from strongest excitation of interneurons to strongest excitation of TC neurons depending on stimulus frequency and duration, and thereby contribute to activity-dependent cortical regulation of thalamocortical transmission between net depression and net facilitation. Studies of postsynaptic response patterns of interneurons to train stimulation demonstrated that cortical input can activate different types of neuronal integration mechanisms that in addition to the STP mechanisms may change the output from dLGN. Lower stimulus intensity, presumably activating few cortical afferents, or moderate frequencies, elicited summation of graded EPSPs reflecting synaptic depression. However, strong activation through higher intensity or frequency, elicited complex response patterns in interneurons caused at least partly by activation of calcium conductances.
Figures




References
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Acuna-Goycolea C, Brenowitz SD, Regehr WG. Active dendritic conductances dynamically regulate GABE release from thalamic interneurons. Neuron. 2008;57:420–431. - PubMed
-
- Ahlsén G, Lindström S, Lo FS. Interaction between inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Exp Brain Res. 1985;58:134–143. - PubMed
-
- Alexander GM, Fisher TL, Godwin DW. Differential response dynamics of corticothalamic glutamatergic synapses in the lateral geniculate nucleus and thalamic reticular nucleus. Neuroscience. 2006;137:367–372. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

