Manifestations and mechanisms of stem cell aging
- PMID: 21502357
- PMCID: PMC3080271
- DOI: 10.1083/jcb.201010131
Manifestations and mechanisms of stem cell aging
Abstract
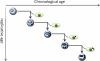
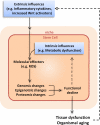
Adult stem cells exist in most mammalian organs and tissues and are indispensable for normal tissue homeostasis and repair. In most tissues, there is an age-related decline in stem cell functionality but not a depletion of stem cells. Such functional changes reflect deleterious effects of age on the genome, epigenome, and proteome, some of which arise cell autonomously and others of which are imposed by an age-related change in the local milieu or systemic environment. Notably, some of the changes, particularly epigenomic and proteomic, are potentially reversible, and both environmental and genetic interventions can result in the rejuvenation of aged stem cells. Such findings have profound implications for the stem cell-based therapy of age-related diseases.
Figures

References
-
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., Rossi D.J. 2010. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA. 107:5465–5470 10.1073/pnas.1000834107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

