Cdk2 loss accelerates precursor differentiation and remyelination in the adult central nervous system
- PMID: 21502361
- PMCID: PMC3080270
- DOI: 10.1083/jcb.201004146
Cdk2 loss accelerates precursor differentiation and remyelination in the adult central nervous system
Abstract
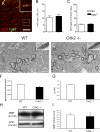
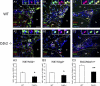
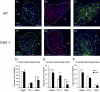
The specific functions of intrinsic regulators of oligodendrocyte progenitor cell (OPC) division are poorly understood. Type 2 cyclin-dependent kinase (Cdk2) controls cell cycle progression of OPCs, but whether it acts during myelination and repair of demyelinating lesions remains unexplored. Here, we took advantage of a viable Cdk2(-/-) mutant mouse to investigate the function of this cell cycle regulator in OPC proliferation and differentiation in normal and pathological conditions. During central nervous system (CNS) development, Cdk2 loss does not affect OPC cell cycle, oligodendrocyte cell numbers, or myelination. However, in response to CNS demyelination, it clearly alters adult OPC renewal, cell cycle exit, and differentiation. Importantly, Cdk2 loss accelerates CNS remyelination of demyelinated axons. Thus, Cdk2 is dispensable for myelination but is important for adult OPC renewal, and could be one of the underlying mechanisms that drive adult progenitors to differentiate and thus regenerate myelin.
Figures





References
-
- Atanasoski S., Boentert M., De Ventura L., Pohl H., Baranek C., Beier K., Young P., Barbacid M., Suter U. 2008. Postnatal Schwann cell proliferation but not myelination is strictly and uniquely dependent on cyclin-dependent kinase 4 (cdk4). Mol. Cell. Neurosci. 37:519–527 10.1016/j.mcn.2007.11.005 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

