Glucose metabolism and cardiac hypertrophy
- PMID: 21502371
- PMCID: PMC3078804
- DOI: 10.1093/cvr/cvr071
Glucose metabolism and cardiac hypertrophy
Abstract
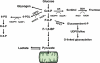
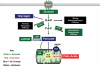
The most notable change in the metabolic profile of hypertrophied hearts is an increased reliance on glucose with an overall reduced oxidative metabolism, i.e. a reappearance of the foetal metabolic pattern. In animal models, this change is attributed to the down-regulation of the transcriptional cascades promoting gene expression for fatty acid oxidation and mitochondrial oxidative phosphorylation in adult hearts. Impaired myocardial energetics in cardiac hypertrophy also triggers AMP-activated protein kinase (AMPK), leading to increased glucose uptake and glycolysis. Aside from increased reliance on glucose as an energy source, changes in other glucose metabolism pathways, e.g. the pentose phosphate pathway, the glucosamine biosynthesis pathway, and anaplerosis, are also noted in the hypertrophied hearts. Studies using transgenic mouse models and pharmacological compounds to mimic or counter the switch of substrate preference in cardiac hypertrophy have demonstrated that increased glucose metabolism in adult heart is not harmful and can be beneficial when it provides sufficient fuel for oxidative metabolism. However, improvement in the oxidative capacity and efficiency rather than the selection of the substrate is likely the ultimate goal for metabolic therapies.
Figures
References
-
- Kresge N, Simoni RD, Hill RL. Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. J Biol Chem. 2005;280:e3. - PubMed
-
- Kornberg H. Krebs and his trinity of cycles. Nat Rev Mol Cell Biol. 2000;1:225–228. - PubMed
-
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. - PubMed
-
- Hasin Y, Barry WH. Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Physiol. 1984;247:H322–H329. - PubMed
-
- Nakamura K, Kusuoka H, Ambrosio G, Becker LC. Glycolysis is necessary to preserve myocardial Ca2+ homeostasis during beta-adrenergic stimulation. Am J Physiol. 1993;264:H670–H678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

