Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma
- PMID: 21502559
- PMCID: PMC3107762
- DOI: 10.1200/JCO.2010.32.9607
Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma
Abstract
Purpose: The purpose of this study was to evaluate the impact of carboplatin and paclitaxel in patients with advanced previously untreated thymoma and thymic carcinoma.
Patients and methods: We conducted a prospective multicenter study in patients with unresectable thymoma (n = 21) or thymic carcinoma (n = 23). Patients were treated with carboplatin (area under the curve, 6) plus paclitaxel (225 mg/m(2)) every 3 weeks for a maximum of six cycles. The primary end point of this trial was to evaluate the objective response rate.
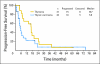
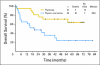
Results: From February 2001 through January 2008, 46 patients were enrolled. Thirteen patients had grade 4 or greater toxicity, mostly neutropenia. Using RECIST (Response Evaluation Criteria in Solid Tumors) 1.0 criteria, three complete responses (CRs) and six partial responses (PRs; objective response rate [ORR], 42.9%; 90% CI, 24.5% to 62.8%) were observed in the thymoma cohort; 10 patients had stable disease. For patients with thymic carcinoma, no CRs and five PRs (ORR, 21.7%; 90% CI, 9.0% to 40.4%) were observed; 12 patients had stable disease. Progression-free survival (PFS) was 16.7 (95% CI, 7.2 to 19.8) and 5.0 (95% CI, 3.0 to 8.3) months for thymoma and thymic carcinoma cohorts, respectively. To date, only seven patients (33.3%) with thymoma have died, compared with 16 patients (69.6%) with thymic carcinoma. Median survival time was 20.0 months (95% CI, 5.0 to 43.6 months) for patients with thymic carcinoma, but it has not been reached for patients with thymoma.
Conclusion: Carboplatin plus paclitaxel has moderate clinical activity for patients with thymic malignancies, but this seems less than expected with anthracycline-based therapy. Patients with thymic carcinoma have poorer PFS and overall survival than patients with thymoma.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: Demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–551. - PubMed
-
- Mullen B, Richardson JD. Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg. 1986;42:338–345. - PubMed
-
- Laurent F, Latrabe V, Lecesne R, et al. Mediastinal masses: Diagnostic approach. Eur Radiol. 1998;8:1148–1159. - PubMed
-
- Verstandig AG, Epstein DM, Miller WT, Jr, et al. Thymoma: Report of 71 cases and a review. Crit Rev Diagn Imaging. 1992;33:201–230. - PubMed
-
- Wilkins EW, Jr, Grillo HC, Scannell JG, et al. J. Maxwell Chamberlain memorial paper: Role of staging in prognosis and management of thymoma. Ann Thorac Surg. 1991;51:888–892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical