A phase II trial of huperzine A in mild to moderate Alzheimer disease
- PMID: 21502597
- PMCID: PMC3269774
- DOI: 10.1212/WNL.0b013e318216eb7b
A phase II trial of huperzine A in mild to moderate Alzheimer disease
Abstract
Objective: Huperzine A is a natural cholinesterase inhibitor derived from the Chinese herb Huperzia serrata that may compare favorably in symptomatic efficacy to cholinesterase inhibitors currently in use for Alzheimer disease (AD).
Methods: We assessed the safety, tolerability, and efficacy of huperzine A in mild to moderate AD in a multicenter trial in which 210 individuals were randomized to receive placebo (n = 70) or huperzine A (200 μg BID [n = 70] or 400 μg BID [n = 70]), for at least 16 weeks, with 177 subjects completing the treatment phase. The primary analysis assessed the cognitive effects of huperzine A 200 μg BID (change in Alzheimer's Disease Assessment Scale-cognitive subscale [ADAS-Cog] at week 16 at 200 μg BID compared to placebo). Secondary analyses assessed the effect of huperzine A 400 μg BID, as well as effect on other outcomes including Mini-Mental State Examination, Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change scale, Alzheimer's Disease Cooperative Study Activities of Daily Living scale, and Neuropsychiatric Inventory (NPI).
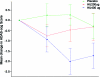
Results: Huperzine A 200 μg BID did not influence change in ADAS-Cog at 16 weeks. In secondary analyses, huperzine A 400 μg BID showed a 2.27-point improvement in ADAS-Cog at 11 weeks vs 0.29-point decline in the placebo group (p = 0.001), and a 1.92-point improvement vs 0.34-point improvement in the placebo arm (p = 0.07) at week 16. Changes in clinical global impression of change, NPI, and activities of daily living were not significant at either dose.
Conclusion: The primary efficacy analysis did not show cognitive benefit with huperzine A 200 μg BID.
Classification of evidence: This study provides Class III evidence that huperzine A 200 μg BID has no demonstrable cognitive effect in patients with mild to moderate AD.
Figures
References
-
- Wang YE, Yue DX, Tang XC. [Anti-cholinesterase activity of huperzine A.] Chung Kuo Yao Li Hsueh Pao 1986;7:110–113 - PubMed
-
- Ashani Y, Peggins JO, Doctor BP. Mechanism of inhibition of cholinesterases by huperzine A. Biochem Biophys Res Commun 1992;184:719–726 - PubMed
-
- Camps P, Muñoz-Torrero D. Tacrine-huperzine A hybrids (huprines): a new class of highly potent and selective acetylcholinesterase inhibitors of interest for the treatment of Alzheimer's disease. Mini Rev Med Chem 2001;1:163–174 - PubMed
-
- Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin 2006;27:1–26 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
