Matrix composition and mechanics of decellularized lung scaffolds
- PMID: 21502745
- PMCID: PMC3696368
- DOI: 10.1159/000324896
Matrix composition and mechanics of decellularized lung scaffolds
Abstract
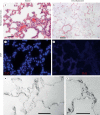
The utility of decellularized native tissues for tissue engineering has been widely demonstrated. Here, we examine the production of decellularized lung scaffolds from native rodent lung using two different techniques, principally defined by use of either the detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) or sodium dodecyl sulfate (SDS). All viable cellular material is removed, including at least 99% of DNA. Histochemical staining and mechanical testing indicate that collagen and elastin are retained in the decellularized matrices with CHAPS-based decellularization, while SDS-based decellularization leads to loss of collagen and decline in mechanical strength. Quantitative assays confirm that most collagen is retained with CHAPS treatment but that about 80% of collagen is lost with SDS treatment. In contrast, for both detergent methods, at least 60% of elastin content is lost along with about 95% of native proteoglycan content. Mechanical testing of the decellularized scaffolds indicates that they are mechanically similar to native lung using CHAPS decellularization, including retained tensile strength and elastic behavior, demonstrating the importance of collagen and elastin in lung mechanics. With SDS decellularization, the mechanical integrity of scaffolds is significantly diminished with some loss of elastic function as well. Finally, a simple theoretical model of peripheral lung matrix mechanics is consonant with our experimental findings. This work demonstrates the feasibility of producing a decellularized lung scaffold that can be used to study lung matrix biology and mechanics, independent of the effects of cellular components.
Copyright © 2011 S. Karger AG, Basel.
Figures




References
-
- Bader A., Schilling T., Teebken O.E., Brandes G., Herden T., Steinhoff G., Haverich A. Tissue engineering of heart valves – human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg. 1998;14:14–279. - PubMed
-
- Badylak S.F., Lantz G.C., Coffey A., Geddes L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:47–74. - PubMed
-
- Bodnar E., Olsen E.G., Florio R., Dobrin J. Damage of porcine aortic valve tissue caused by the surfactant sodiumdodecylsulphate. Thorac Cardiovasc Surg. 1986;34:34–82. - PubMed
-
- Buschmann M.D., Grodzinsky A.J. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:117–179. - PubMed

