Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects
- PMID: 21503667
- PMCID: PMC3163795
- DOI: 10.1007/s00467-011-1871-4
Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects
Abstract
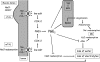
Salt-losing tubulopathies with secondary hyperaldosteronism (SLT) comprise a set of well-defined inherited tubular disorders. Two segments along the distal nephron are primarily involved in the pathogenesis of SLTs: the thick ascending limb of Henle's loop, and the distal convoluted tubule (DCT). The functions of these pre- and postmacula densa segments are quite distinct, and this has a major impact on the clinical presentation of loop and DCT disorders - the Bartter- and Gitelman-like syndromes. Defects in the water-impermeable thick ascending limb, with its greater salt reabsorption capacity, lead to major salt and water losses similar to the effect of loop diuretics. In contrast, defects in the DCT, with its minor capacity of salt reabsorption and its crucial role in fine-tuning of urinary calcium and magnesium excretion, provoke more chronic solute imbalances similar to the effects of chronic treatment with thiazides. The most severe disorder is a combination of a loop and DCT disorder similar to the enhanced diuretic effect of a co-medication of loop diuretics with thiazides. Besides salt and water supplementation, prostaglandin E2-synthase inhibition is the most effective therapeutic option in polyuric loop disorders (e.g., pure furosemide and mixed furosemide-amiloride type), especially in preterm infants with severe volume depletion. In DCT disorders (e.g., pure thiazide and mixed thiazide-furosemide type), renin-angiotensin-aldosterone system (RAAS) blockers might be indicated after salt, potassium, and magnesium supplementation are deemed insufficient. It appears that in most patients with SLT, a combination of solute supplementation with some drug treatment (e.g., indomethacin) is needed for a lifetime.
Figures

Comment in
-
Acetyl salicylic acid treatment in neonatal Bartter syndrome--a commentary letter.Pediatr Nephrol. 2011 Aug;26(8):1341-2. doi: 10.1007/s00467-011-1922-x. Epub 2011 May 31. Pediatr Nephrol. 2011. PMID: 21626220 Free PMC article. No abstract available.
References
-
- Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274:R263–R279. - PubMed
-
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

