Functional redundancy of type II BMP receptor and type IIB activin receptor in BMP2-induced osteoblast differentiation
- PMID: 21503889
- PMCID: PMC3684695
- DOI: 10.1002/jcp.22802
Functional redundancy of type II BMP receptor and type IIB activin receptor in BMP2-induced osteoblast differentiation
Abstract
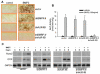
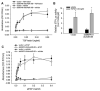
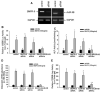
Signaling pathways for bone morphogenetic proteins (BMPs) are important in osteoblast differentiation. Although the precise function of type I BMP receptors in mediating BMP signaling for osteoblast differentiation and bone formation has been characterized previously, the role of type II BMP receptors in osteoblasts is to be well clarified. In this study, we investigated the role of type II BMP receptor (BMPR-II) and type IIB activin receptor (ActR-IIB) in BMP2-induced osteoblast differentiation. While osteoblastic 2T3 cells expressed BMPR-II and ActR-IIB, loss-of-function studies, using dominant negative receptors and siRNAs, showed that BMPR-II and ActR-IIB compensated each other functionally in mediating BMP2 signaling and BMP2-induced osteoblast differentiation. This was evidenced by two findings. First, unless there was loss of function of both type II receptors, isolated disruption of either BMPR-II or ActR-IIB did not remove BMP2 activity. Second, in cells with loss of function of both receptors, restoration of function of either BMPR-II or ActR-IIB by transfection of the wild-type forms, restored BMP2 activity. These findings suggest a functional redundancy between BMPR-II and ActR-IIB in osteoblast differentiation. Results from experiments to test the effects of transforming growth factor β (TGF-β), activin, and fibroblast growth factor (FGF) on osteoblast proliferation and differentiation suggest that inhibition of receptor signaling by double-blockage of BMPR-II and ActR-IIB is BMP-signaling specific. The observed functional redundancy of type II BMP receptors in osteoblasts is novel information about the BMP signaling pathway essential for initiating osteoblast differentiation.
Copyright © 2011 Wiley Periodicals, Inc.
Figures


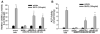
Similar articles
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation.J Cell Sci. 1999 Oct;112 ( Pt 20):3519-27. doi: 10.1242/jcs.112.20.3519. J Cell Sci. 1999. PMID: 10504300
-
A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor.BMC Struct Biol. 2007 Feb 12;7:6. doi: 10.1186/1472-6807-7-6. BMC Struct Biol. 2007. PMID: 17295905 Free PMC article.
-
Differentiation of murine preosteoblastic KS483 cells depends on autocrine bone morphogenetic protein signaling during all phases of osteoblast formation.Bone. 2002 Dec;31(6):661-9. doi: 10.1016/s8756-3282(02)00903-1. Bone. 2002. PMID: 12531559
-
The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S7-14. J Bone Joint Surg Am. 2001. PMID: 11263668 Review.
Cited by
-
The genetic pleiotropy of musculoskeletal aging.Front Physiol. 2012 Aug 8;3:303. doi: 10.3389/fphys.2012.00303. eCollection 2012. Front Physiol. 2012. PMID: 22934054 Free PMC article.
-
Cytochrome P450 1B1: A Key Regulator of Ocular Iron Homeostasis and Oxidative Stress.Cells. 2022 Sep 20;11(19):2930. doi: 10.3390/cells11192930. Cells. 2022. PMID: 36230892 Free PMC article. Review.
-
BMP Ligand Trap ALK3-Fc Attenuates Osteogenesis and Heterotopic Ossification in Blast-Related Lower Extremity Trauma.Stem Cells Dev. 2021 Jan 15;30(2):91-105. doi: 10.1089/scd.2020.0162. Epub 2020 Dec 24. Stem Cells Dev. 2021. PMID: 33256557 Free PMC article.
-
BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics.PLoS Biol. 2019 Dec 11;17(12):e3000557. doi: 10.1371/journal.pbio.3000557. eCollection 2019 Dec. PLoS Biol. 2019. PMID: 31826007 Free PMC article.
-
Loss of BMPR2 leads to high bone mass due to increased osteoblast activity.J Cell Sci. 2015 Apr 1;128(7):1308-15. doi: 10.1242/jcs.156737. Epub 2015 Feb 6. J Cell Sci. 2015. PMID: 25663702 Free PMC article.
References
-
- Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1241–L1247. - PubMed
-
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. - PubMed
-
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

