Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells
- PMID: 21503965
- PMCID: PMC3155646
- DOI: 10.1002/jcb.23150
Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells
Retraction in
-
Retraction: "Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells" by Bao et al.J Cell Biochem. 2016 Aug;117(8):1963. doi: 10.1002/jcb.25588. J Cell Biochem. 2016. PMID: 27301890 Free PMC article.
Abstract
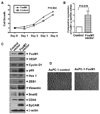
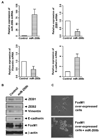
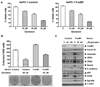
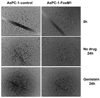
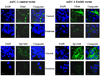
FoxM1 is known to play important role in the development and progression of many malignancies including pancreatic cancer. Studies have shown that the acquisition of epithelial-to-mesenchymal transition (EMT) phenotype and induction of cancer stem cell (CSC) or cancer stem-like cell phenotypes are highly inter-related, and contributes to drug resistance, tumor recurrence, and metastasis. The molecular mechanism(s) by which FoxM1 contributes to the acquisition of EMT phenotype and induction of CSC self-renewal capacity is poorly understood. Therefore, we established FoxM1 over-expressing pancreatic cancer (AsPC-1) cells, which showed increased cell growth, clonogenicity, and cell migration. Moreover, over-expression of FoxM1 led to the acquisition of EMT phenotype by activation of mesenchymal cell markers, ZEB1, ZEB2, Snail2, E-cadherin, and vimentin, which is consistent with increased sphere-forming (pancreatospheres) capacity and expression of CSC surface markers (CD44 and EpCAM). We also found that over-expression of FoxM1 led to decreased expression of miRNAs (let-7a, let-7b, let-7c, miR-200b, and miR-200c); however, re-expression of miR-200b inhibited the expression of ZEB1, ZEB2, vimentin as well as FoxM1, and induced the expression of E-cadherin, leading to the reversal of EMT phenotype. Finally, we found that genistein, a natural chemo-preventive agent, inhibited cell growth, clonogenicity, cell migration and invasion, EMT phenotype, and formation of pancreatospheres consistent with reduced expression of CD44 and EpCAM. These results suggest, for the first time, that FoxM1 over-expression is responsible for the acquisition of EMT and CSC phenotype, which is in part mediated through the regulation of miR-200b and these processes, could be easily attenuated by genistein.
Copyright © 2011 Wiley-Liss, Inc.
Figures


References
-
- Bindels S, Mestdagt M, Vandewalle C, Jacobs N, Volders L, Noel A, Van RF, Berx G, Foidart JM, Gilles C. Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene. 2006;25:4975–4985. - PubMed
-
- Cano A, Nieto MA. Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol. 2008;18:357–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

