Carbohydrate polymers for nonviral nucleic acid delivery
- PMID: 21504102
- PMCID: PMC4096969
- DOI: 10.1007/128_2010_68
Carbohydrate polymers for nonviral nucleic acid delivery
Abstract
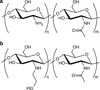
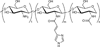
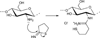
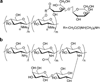
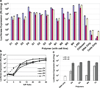
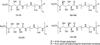
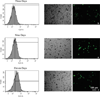
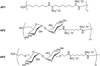
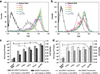
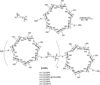
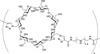
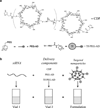
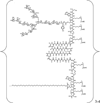
Carbohydrates have been investigated and developed as delivery vehicles for shuttling nucleic acids into cells. In this review, we present the state of the art in carbohydrate-based polymeric vehicles for nucleic acid delivery, with the focus on the recent successes in preclinical models, both in vitro and in vivo. Polymeric scaffolds based on the natural polysaccharides chitosan, hyaluronan, pullulan, dextran, and schizophyllan each have unique properties and potential for modification, and these results are discussed with the focus on facile synthetic routes and favorable performance in biological systems. Many of these carbohydrates have been used to develop alternative types of biomaterials for nucleic acid delivery to typical polyplexes, and these novel materials are discussed. Also presented are polymeric vehicles that incorporate copolymerized carbohydrates into polymer backbones based on polyethylenimine and polylysine and their effect on transfection and biocompatibility. Unique scaffolds, such as clusters and polymers based on cyclodextrin (CD), are also discussed, with the focus on recent successes in vivo and in the clinic. These results are presented with the emphasis on the role of carbohydrate and charge on transfection. Use of carbohydrates as molecular recognition ligands for cell-type specific delivery is also briefly reviewed. We contend that carbohydrates have contributed significantly to progress in the field of non-viral DNA delivery, and these new discoveries are impactful for developing new vehicles and materials for treatment of human disease.
Figures


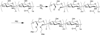

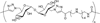

Similar articles
-
Enhancing the In Vitro and In Vivo Stabilities of Polymeric Nucleic Acid Delivery Nanosystems.Bioconjug Chem. 2019 Feb 20;30(2):325-337. doi: 10.1021/acs.bioconjchem.8b00749. Epub 2018 Dec 28. Bioconjug Chem. 2019. PMID: 30592619 Free PMC article. Review.
-
pH-Sensitive Polymers as Dynamic Mediators of Barriers to Nucleic Acid Delivery.Bioconjug Chem. 2019 Feb 20;30(2):350-365. doi: 10.1021/acs.bioconjchem.8b00695. Epub 2018 Nov 15. Bioconjug Chem. 2019. PMID: 30398844 Review.
-
Mixing-sequence-dependent nucleic acid complexation and gene transfer efficiency by polyethylenimine.Biomater Sci. 2015 Jul;3(7):1124-33. doi: 10.1039/c5bm00041f. Epub 2015 Mar 18. Biomater Sci. 2015. PMID: 26221945
-
Poly(glycoamidoamine)s: a broad class of carbohydrate-containing polycations for nucleic acid delivery.Trends Biotechnol. 2011 Sep;29(9):443-53. doi: 10.1016/j.tibtech.2011.04.012. Epub 2011 Jun 24. Trends Biotechnol. 2011. PMID: 21705101 Review.
-
Current Status and Trends in Nucleic Acids for Cancer Therapy: A Focus on Polysaccharide-Based Nanomedicines.Macromol Biosci. 2023 Sep;23(9):e2300102. doi: 10.1002/mabi.202300102. Epub 2023 Jun 3. Macromol Biosci. 2023. PMID: 37212473 Review.
Cited by
-
From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape.Front Immunol. 2021 Jul 7;12:679344. doi: 10.3389/fimmu.2021.679344. eCollection 2021. Front Immunol. 2021. PMID: 34305909 Free PMC article. Review.
-
Cationic starch/pDNA nanocomplexes assembly and their nanostructure changes on gene transfection efficiency.Sci Rep. 2017 Nov 1;7(1):14844. doi: 10.1038/s41598-017-14551-1. Sci Rep. 2017. PMID: 29093552 Free PMC article.
-
Endocytic Profiling of Cancer Cell Models Reveals Critical Factors Influencing LNP-Mediated mRNA Delivery and Protein Expression.Mol Ther. 2019 Nov 6;27(11):1950-1962. doi: 10.1016/j.ymthe.2019.07.018. Epub 2019 Aug 5. Mol Ther. 2019. PMID: 31427168 Free PMC article.
-
Cationic glycopolymers for the delivery of pDNA to human dermal fibroblasts and rat mesenchymal stem cells.Biomaterials. 2012 Feb;33(6):1851-62. doi: 10.1016/j.biomaterials.2011.10.031. Epub 2011 Dec 3. Biomaterials. 2012. PMID: 22138032 Free PMC article.
-
Investigating the effects of block versus statistical glycopolycations containing primary and tertiary amines for plasmid DNA delivery.Biomacromolecules. 2014 Jul 14;15(7):2616-28. doi: 10.1021/bm5004527. Epub 2014 Jun 20. Biomacromolecules. 2014. PMID: 24901035 Free PMC article.
References
-
- Pickler RH, Munro CL. Gene therapy for inherited disorders. J Pediatr Nurs. 1995;10:40–47. - PubMed
-
- Anderson WF. Human gene therapy. Science. 1992;256:803–813.
-
- Mulligan RC. The basic science of gene therapy. Science. 1993;260:926–932. - PubMed
-
- Wiethoff CM, Middaugh CR. Barriers to nonviral gene delivery. J Pharm Sci. 2003;92(2):203–217. - PubMed
-
- Braun CS, Vetro JA, Tomalia DA, et al. Structure/function relationships of polyamidoamine/ DNA dendrimers as gene delivery vehicles. J Pharm Sci. 2005;94(2):423–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

