Cationic liposome-nucleic acid complexes for gene delivery and silencing: pathways and mechanisms for plasmid DNA and siRNA
- PMID: 21504103
- PMCID: PMC3867951
- DOI: 10.1007/128_2010_70
Cationic liposome-nucleic acid complexes for gene delivery and silencing: pathways and mechanisms for plasmid DNA and siRNA
Abstract
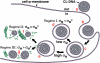
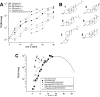
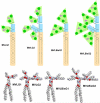
Motivated by the promises of gene therapy, there is great interest in developing non-viral lipid-based vectors for therapeutic applications due to their low immunogenicity, low toxicity, ease of production, and the potential of transferring large pieces of DNA into cells. In fact, cationic liposome (CL) based vectors are among the prevalent synthetic carriers of nucleic acids (NAs) currently used in gene therapy clinical trials worldwide. These vectors are studied both for gene delivery with CL-DNA complexes and gene silencing with CL-siRNA (short interfering RNA) complexes. However, their transfection efficiencies and silencing efficiencies remain low compared to those of engineered viral vectors. This reflects the currently poor understanding of transfection-related mechanisms at the molecular and self-assembled levels, including a lack of knowledge about interactions between membranes and double stranded NAs and between CL-NA complexes and cellular components. In this review we describe our recent efforts to improve the mechanistic understanding of transfection by CL-NA complexes, which will help to design optimal lipid-based carriers of DNA and siRNA for therapeutic gene delivery and gene silencing.
Figures
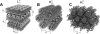
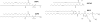

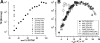
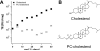


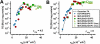


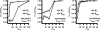
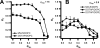



References
-
- Safinya CR, Koltover I. Self Assembled Structures of Lipid–DNA Nonviral Gene Delivery Systems from Synchrotron X-Ray Diffraction. In: Huang L, Hung M-C, Wagner E, editors. Non-Viral Vectors for Gene Therapy. Academic Press; San Diego: 1999.
-
- Ewert KK, Slack NL, Ahmad A, Evans HM, Lin A, Samuel CE, Safinya CR. Cationic Lipid–DNA Complexes for Gene Therapy: Understanding the Relationship between Complex Structures and Gene Delivery Pathways at the Molecular Level. Curr Med Chem. 2004;11:1241–1253. - PubMed
-
- Ewert K, Evans H, Ahmad A, Slack L, Lin A, Martin-Herranz A, Safinya CR. Lipoplex Structures and their Distinct Cellular Pathways. In: Huang L, Hung M-C, Wagner E, editors. Non-Viral Vectors for Gene Therapy. 2nd edn. Elsevier; San Diego: 2005. Part I (Advances in Genetics 53)
-
- Ewert K, Ahmad A, Evans H, Safinya CR. Cationic lipid–DNA complexes for non-viral gene therapy: relating supramolecular structures to cellular pathways. Expert Opin Biol Ther. 2005;5:33–53. - PubMed
-
- Safinya CR, Ewert KK, Ahmad A, Evans HM, Raviv U, Needleman DJ, Lin AJ, Slack NL, George CX, Samuel CE. Cationic Liposome–DNA Complexes: From Liquid Crystal Science to Gene Delivery Applications. Phil Transact Royal Soc A. 2006;364:2573–2596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

