Drug discovery of antimicrobial photosensitizers using animal models
- PMID: 21504410
- PMCID: PMC3463379
- DOI: 10.2174/138161211795703735
Drug discovery of antimicrobial photosensitizers using animal models
Abstract
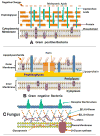
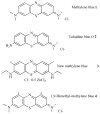
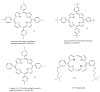
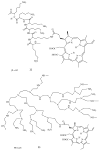
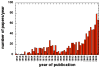
Antimicrobial photodynamic therapy (aPDT) is an emerging alternative to antibiotics motivated by growing problems with multi-drug resistant pathogens. aPDT uses non-toxic dyes or photosensitizers (PS) in combination with harmless visible of the correct wavelength to be absorbed by the PS. The excited state PS can form a long-lived triplet state that can interact with molecular oxygen to produce reactive oxygen species such as singlet oxygen and hydroxyl radical that kill the microbial cells. To obtain effective PS for treatment of infections it is necessary to use cationic PS with positive charges that are able to bind to and penetrate different classes of microbial cells. Other drug design criteria require PS with high absorption coefficients in the red/near infra-red regions of the spectrum where light penetration into tissue is maximum, high photostability to minimize photobleaching, and devising compounds that will selectively bind to microbial cells rather than host mammalian cells. Several molecular classes fulfill many of these requirements including phenothiazinium dyes, cationic tetrapyrroles such as porphyrins, phthalocyanines and bacteriochlorins, cationic fullerenes and cationic derivatives of other known PS. Larger structures such as conjugates between PS and cationic polymers, cationic nanoparticles and cationic liposomes that contain PS are also effective. In order to demonstrate in vivo efficacy it is necessary to use animal models of localized infections in which both PS and light can be effectively delivered into the infected area. This review will cover a range of mouse models we have developed using bioluminescent pathogens and a sensitive low light imaging system to non-invasively monitor the progress of the infection in real time. Effective aPDT has been demonstrated in acute lethal infections and chronic biofilm infections; in infections caused by Gram-positive, Gram-negative bacteria and fungi; in infections in wounds, third degree burns, skin abrasions and soft-tissue abscesses. This range of animal models also represents a powerful aid in antimicrobial drug discovery.
Figures






References
-
- Levy JG, Obochi M. New applications in photodynamic therapy. Introduction Photochem Photobiol. 1996;64:737–9. - PubMed
-
- Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. - PubMed
-
- Raab O. Über die Wirkung fluoresceinder Stoffe and Infusorien. Zeit für Biol. 1900;39:524–546.
-
- Wainwright W. Photoantimicrobials - a PACT against resistance and infection. Drug Future. 2004;29:85–93.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

