A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart
- PMID: 21504733
- PMCID: PMC3077696
- DOI: 10.1016/j.bpj.2011.02.061
A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart
Abstract
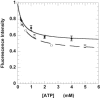
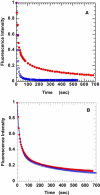
The mechanisms that control cardiac contractility are complex. Recent work we conducted in vertebrate skeletal muscle identified a new state of myosin, the super-relaxed state (SRX), which had a very low metabolic rate. To determine whether this state also exists in cardiac muscle we used quantitative epi-fluorescence to measure single nucleotide turnovers by myosin in bundles of relaxed permeable rabbit ventricle cells. We measured two turnover times--one compatible with the normal relaxed state, and one much slower which was shown to arise from myosin heads in the SRX. In both skeletal and cardiac muscle, the SRX appears to play a similar role in relaxed cells, providing a state with a very low metabolic rate. However, in active muscle the properties of the SRX differ dramatically. We observed a rapid transition of myosin heads out of the SRX in active skeletal fibers, whereas the population of the SRX remained constant in active cardiac cells. This property allows the SRX to play a very different role in cardiac muscle than in skeletal muscle. The SRX could provide a mechanism for decreasing the metabolic load on the heart, being cardioprotective, particularly in time of stress such as ischemia.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

