Golgi positioning
- PMID: 21504874
- PMCID: PMC3101843
- DOI: 10.1101/cshperspect.a005322
Golgi positioning
Abstract
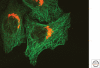
The Golgi apparatus in mammalian cells is positioned near the centrosome-based microtubule-organizing center (Fig. 1). Secretory cargo moves inward in membrane carriers for delivery to Golgi membranes in which it is processed and packaged for transport outward to the plasma membrane. Cytoplasmic dynein motor proteins (herein termed dynein) primarily mediate inward cargo carrier movement and Golgi positioning. These motors move along microtubules toward microtubule minus-ends embedded in centrosomes. Centripetal motility is controlled by a host of regulators whose precise functions remain to be determined. Significantly, a specific Golgi receptor for dynein has not been identified. This has impaired progress toward elucidation of membrane-motor-microtubule attachment in the periphery and, after inward movement, recycling of the motor for another round. Pericentrosomal positioning of the Golgi apparatus is dynamic. It is regulated during critical cellular processes such as mitosis, differentiation, cell polarization, and cell migration. Positioning is also important as it aligns the Golgi along an axis of cell polarity. In certain cell types, this promotes secretion directed to the proximal plasma membrane domain thereby maintaining specializations critical for diverse processes including wound healing, immunological synapse formation, and axon determination.
Figures






References
-
- Addinall SG, Mayr PS, Doyle S, Sheehan JK, Woodman PG, Allan VJ 2001. Phosphorylation by cdc2-CyclinB1 kinase releases cytoplasmic dynein from membranes. J Biol Chem 276: 15939–15944 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
