Embryonic toxin expression in the cone snail Conus victoriae: primed to kill or divergent function?
- PMID: 21504902
- PMCID: PMC3121399
- DOI: 10.1074/jbc.M110.217703
Embryonic toxin expression in the cone snail Conus victoriae: primed to kill or divergent function?
Abstract
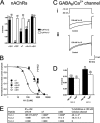
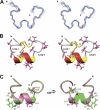
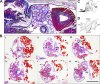
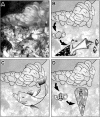
Predatory marine cone snails (genus Conus) utilize complex venoms mainly composed of small peptide toxins that target voltage- and ligand-gated ion channels in their prey. Although the venoms of a number of cone snail species have been intensively profiled and functionally characterized, nothing is known about the initiation of venom expression at an early developmental stage. Here, we report on the expression of venom mRNA in embryos of Conus victoriae and the identification of novel α- and O-conotoxin sequences. Embryonic toxin mRNA expression is initiated well before differentiation of the venom gland, the organ of venom biosynthesis. Structural and functional studies revealed that the embryonic α-conotoxins exhibit the same basic three-dimensional structure as the most abundant adult toxin but significantly differ in their neurological targets. Based on these findings, we postulate that the venom repertoire of cone snails undergoes ontogenetic changes most likely reflecting differences in the biotic interactions of these animals with their prey, predators, or competitors. To our knowledge, this is the first study to show toxin mRNA transcripts in embryos, a finding that extends our understanding of the early onset of venom expression in animals and may suggest alternative functions of peptide toxins during development.
Figures



References
-
- Terlau H., Olivera B. M. (2004) Physiol. Rev. 84, 41–68 - PubMed
-
- Olivera B. M. (2006) J. Biol. Chem. 281, 31173–31177 - PubMed
-
- Norton R. S., Olivera B. M. (2006) Toxicon 48, 780–798 - PubMed
-
- Pisarewicz K., Mora D., Pflueger F. C., Fields G. B., Marí F. (2005) J. Am. Chem. Soc. 127, 6207–6215 - PubMed
-
- Lopez-Vera E., Walewska A., Skalicky J. J., Olivera B. M., Bulaj G. (2008) Biochemistry 47, 1741–1751 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

