The tumor suppressor gene Trp53 protects the mouse lens against posterior subcapsular cataracts and the BMP receptor Acvr1 acts as a tumor suppressor in the lens
- PMID: 21504908
- PMCID: PMC3124053
- DOI: 10.1242/dmm.006593
The tumor suppressor gene Trp53 protects the mouse lens against posterior subcapsular cataracts and the BMP receptor Acvr1 acts as a tumor suppressor in the lens
Abstract
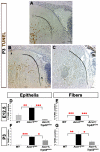
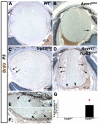
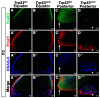
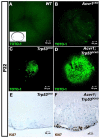
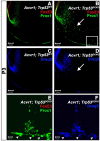
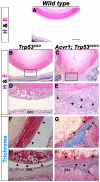
We previously found that lenses lacking the Acvr1 gene, which encodes a bone morphogenetic protein (BMP) receptor, had abnormal proliferation and cell death in epithelial and cortical fiber cells. We tested whether the tumor suppressor protein p53 (encoded by Trp53) affected this phenotype. Acvr1 conditional knockout (Acvr1(CKO)) mouse fiber cells had increased numbers of nuclei that stained for p53 phosphorylated on serine 15, an indicator of p53 stabilization and activation. Deletion of Trp53 rescued the Acvr1(CKO) cell death phenotype in embryos and reduced Acvr1-dependent apoptosis in postnatal lenses. However, deletion of Trp53 alone increased the number of fiber cells that failed to withdraw from the cell cycle. Trp53(CKO) and Acvr1;Trp53(DCKO) (double conditional knockout), but not Acvr1(CKO), lenses developed abnormal collections of cells at the posterior of the lens that resembled posterior subcapsular cataracts. Cells from human posterior subcapsular cataracts had morphological and molecular characteristics similar to the cells at the posterior of mouse lenses lacking Trp53. In Trp53(CKO) lenses, cells in the posterior plaques did not proliferate but, in Acvr1;Trp53(DCKO) lenses, many cells in the posterior plaques continued to proliferate, eventually forming vascularized tumor-like masses at the posterior of the lens. We conclude that p53 protects the lens against posterior subcapsular cataract formation by suppressing the proliferation of fiber cells and promoting the death of any fiber cells that enter the cell cycle. Acvr1 acts as a tumor suppressor in the lens. Enhancing p53 function in the lens could contribute to the prevention of steroid- and radiation-induced posterior subcapsular cataracts.
Figures




References
-
- Achison M., Hupp T. R. (2003). Hypoxia attenuates the p53 response to cellular damage. Oncogene 22, 3431–3440 - PubMed
-
- Almog N., Rotter V. (1997). Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta. 1333, F1–F27 - PubMed
-
- Atfi A., Baron R. (2008). p53 brings a new twist to the Smad signaling network. Sci. Signal. 1, pe33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

