D-myo-inositol-3-phosphate affects phosphatidylinositol-mediated endomembrane function in Arabidopsis and is essential for auxin-regulated embryogenesis
- PMID: 21505066
- PMCID: PMC3101546
- DOI: 10.1105/tpc.111.083337
D-myo-inositol-3-phosphate affects phosphatidylinositol-mediated endomembrane function in Arabidopsis and is essential for auxin-regulated embryogenesis
Abstract
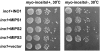
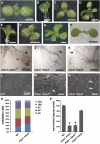
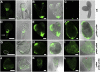
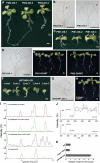
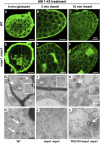
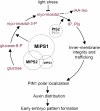
In animal cells, myo-inositol is an important regulatory molecule in several physiological and biochemical processes, including signal transduction and membrane biogenesis. However, the fundamental biological functions of myo-inositol are still far from clear in plants. Here, we report the genetic characterization of three Arabidopsis thaliana genes encoding D-myo-inositol-3-phosphate synthase (MIPS), which catalyzes the rate-limiting step in de novo synthesis of myo-inositol. Each of the three MIPS genes rescued the yeast ino1 mutant, which is defective in yeast MIPS gene INO1, and they had different dynamic expression patterns during Arabidopsis embryo development. Although single mips mutants showed no obvious phenotypes, the mips1 mips2 double mutant and the mips1 mips2 mips3 triple mutant were embryo lethal, whereas the mips1 mips3 and mips1 mips2⁺/⁻ double mutants had abnormal embryos. The mips phenotypes resembled those of auxin mutants. Indeed, the double and triple mips mutants displayed abnormal expression patterns of DR5:green fluorescent protein, an auxin-responsive fusion protein, and they had altered PIN1 subcellular localization. Also, membrane trafficking was affected in mips1 mips3. Interestingly, overexpression of PHOSPHATIDYLINOSITOL SYNTHASE2, which converts myo-inositol to membrane phosphatidylinositol (PtdIns), largely rescued the cotyledon and endomembrane defects in mips1 mips3. We conclude that myo-inositol serves as the main substrate for synthesizing PtdIns and phosphatidylinositides, which are essential for endomembrane structure and trafficking and thus for auxin-regulated embryogenesis.
Figures
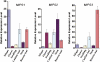





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

