C.E.R.A. once every 4 weeks corrects anaemia and maintains haemoglobin in patients with chronic kidney disease not on dialysis
- PMID: 21505096
- PMCID: PMC3224113
- DOI: 10.1093/ndt/gfr160
C.E.R.A. once every 4 weeks corrects anaemia and maintains haemoglobin in patients with chronic kidney disease not on dialysis
Abstract
Background: No previous randomized controlled studies have been reported examining de novo, once every 4 weeks (Q4W) administration of erythropoiesis-stimulating agents in chronic kidney disease (CKD) patients. We report results from a randomized multinational study that compared continuous erythropoietin receptor activator (C.E.R.A.) Q4W with darbepoetin alfa once weekly (QW) or every 2 weeks (Q2W) for the correction of anaemia in non-dialysis CKD patients.
Methods: Patients were randomized (1:1) to receive either 1.2 μg/kg C.E.R.A. Q4W or darbepoetin alfa QW/Q2W during a 20-week correction period and an 8-week evaluation period. Two primary end points were assessed: the haemoglobin (Hb) response rate and the change in average Hb concentration between baseline and evaluation.
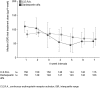
Results: The Hb response rate for C.E.R.A. was 94.1%, significantly higher than the protocol-specified 60% response rate [95% confidence interval (CI): 89.1, 97.3; P < 0.0001] and comparable with darbepoetin alfa (93.5%; 95% CI: 88.4, 96.8; P < 0.0001). C.E.R.A. Q4W was non-inferior to darbepoetin alfa QW/Q2W, with similar mean Hb changes from baseline of 1.62 g/dL and 1.66 g/dL, respectively. Patients receiving C.E.R.A. showed a steady rise in Hb, with fewer patients above the target range during the first 8 weeks compared with darbepoetin alfa [39 patients (25.8%) versus 72 patients (47.7%); P < 0.0001]. Adverse event rates were comparable between the treatment groups.
Conclusion: C.E.R.A. Q4W successfully corrects anaemia and maintains stable Hb levels within the recommended target range in non-dialysis CKD patients.
Figures
References
-
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. - PubMed
-
- Lefebvre P, Vekeman F, Sarokhan B, et al. Relationship between hemoglobin level and quality of life in anemic patients with chronic kidney disease receiving epoetin alfa. Curr Med Res Opin. 2006;22:1929–1937. - PubMed
-
- Halstenson CE, Macres M, Katz SA, et al. Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther. 1991;50:702–712. - PubMed
-
- Jarsch M, Brandt M, Lanzendörfer M, et al. Comparative erythropoietin receptor binding kinetics of C.E.R.A. and epoetin-beta determined by surface plasmon resonance and competition binding assay. Pharmacology. 2008;81:63–69. - PubMed
-
- Macdougall IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–2395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical