FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion
- PMID: 21505104
- PMCID: PMC3108023
- DOI: 10.1158/0008-5472.CAN-10-2603
FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion
Abstract
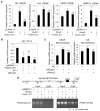
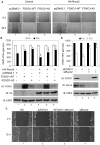
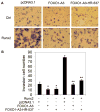
Prostate cancer patients with regional lymph node involvement at radical prostatectomy often experience disease progression to other organs, with the bone as the predominant site. The transcription factor Runx2 plays an important role in bone formation and prostate cancer cell migration, invasion, and metastasis. Here we showed that the forkhead box O (FOXO1) protein, a key downstream effector of the tumor suppressor PTEN, inhibits the transcriptional activity of Runx2 in prostate cancer cells. This inhibition was enhanced by PTEN but diminished by active Akt. FOXO1 bound to Runx2 in vitro and in vivo and suppressed Runx2's activity independent of its transcriptional function. FOXO1 inhibited Runx2-promoted migration of prostate cancer cells, whereas silencing of endogenous FOXO1 enhanced prostate cancer cell migration in a Runx2-dependent manner. Forced expression of FOXO1 also inhibited Runx2-promoted prostate cancer cell invasion. Finally, we found that expression of PTEN and the level of FOXO1 in the nucleus is inversely correlated with expression of Runx2 in a cohort of prostate cancer specimens from patients with lymph node and bone metastasis. These data reveal FOXO1 as a critical negative regulator of Runx2 in prostate cancer cells. Inactivation of FOXO1 due to frequent loss of PTEN in prostate cancer cells may leave the oncogenic activities of Runx2 unchecked, thereby driving promiscuous expression of Runx2 target genes involved in cell migration and invasion and favoring prostate cancer progression.
Figures



Comment in
-
Prostate cancer: molecular insights into bone metastasis.Nat Rev Urol. 2011 Jun 10;8(6):291. doi: 10.1038/nrurol.2011.71. Nat Rev Urol. 2011. PMID: 21660068 No abstract available.
Similar articles
-
Inhibition of FOXO1/3 promotes vascular calcification.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):175-83. doi: 10.1161/ATVBAHA.114.304786. Epub 2014 Nov 6. Arterioscler Thromb Vasc Biol. 2015. PMID: 25378413 Free PMC article.
-
A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells.Cancer Res. 2008 Dec 15;68(24):10290-9. doi: 10.1158/0008-5472.CAN-08-2038. Cancer Res. 2008. PMID: 19074897
-
Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts.J Biol Chem. 2011 May 27;286(21):19149-58. doi: 10.1074/jbc.M110.197905. Epub 2011 Apr 6. J Biol Chem. 2011. PMID: 21471200 Free PMC article.
-
Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer.Adv Exp Med Biol. 2019;1210:319-331. doi: 10.1007/978-3-030-32656-2_14. Adv Exp Med Biol. 2019. PMID: 31900915 Review.
-
Metastatic bone disease: role of transcription factors and future targets.Bone. 2011 Jan;48(1):30-6. doi: 10.1016/j.bone.2010.05.035. Epub 2010 Jun 1. Bone. 2011. PMID: 20561908 Free PMC article. Review.
Cited by
-
Altered miRNAs Expression Correlates With Gastroenteropancreatic Neuroendocrine Tumors Grades.Front Oncol. 2020 Jul 17;10:1187. doi: 10.3389/fonc.2020.01187. eCollection 2020. Front Oncol. 2020. PMID: 32766159 Free PMC article.
-
Decreased FOXO1 Expression Is Correlated with Poor Prognosis in Myelodysplastic Syndromes.Curr Oncol. 2022 Sep 25;29(10):6933-6946. doi: 10.3390/curroncol29100545. Curr Oncol. 2022. PMID: 36290822 Free PMC article.
-
Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1.PLoS One. 2013 Aug 12;8(8):e72400. doi: 10.1371/journal.pone.0072400. eCollection 2013. PLoS One. 2013. PMID: 23951320 Free PMC article.
-
FOXO transcription factor family in cancer and metastasis.Cancer Metastasis Rev. 2020 Sep;39(3):681-709. doi: 10.1007/s10555-020-09883-w. Cancer Metastasis Rev. 2020. PMID: 32372224 Free PMC article. Review.
-
MET promotes hepatocellular carcinoma development through the promotion of TRIB3-mediated FOXO1 degradation.Clin Mol Hepatol. 2025 Jul;31(3):1032-1057. doi: 10.3350/cmh.2024.1163. Epub 2025 Apr 11. Clin Mol Hepatol. 2025. PMID: 40211872 Free PMC article.
References
-
- Deocampo ND, Huang H, Tindall DJ. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003;28:145–53. - PubMed
-
- Feilotter HE, Nagai MA, Boag AH, Eng C, Mulligan LM. Analysis of PTEN and the 10q23 region in primary prostate carcinomas. Oncogene. 1998;16:1743–8. - PubMed
-
- Suzuki H, Freije D, Nusskern DR, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–9. - PubMed
-
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

