Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer
- PMID: 21505105
- PMCID: PMC3085609
- DOI: 10.1158/0008-5472.CAN-10-4102
Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer
Abstract
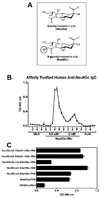
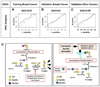
Human carcinomas can metabolically incorporate and present the dietary non-human sialic acid Neu5Gc, which differs from the human sialic acid N-acetylneuraminic acid (Neu5Ac) by 1 oxygen atom. Tumor-associated Neu5Gc can interact with low levels of circulating anti-Neu5Gc antibodies, thereby facilitating tumor progression via chronic inflammation in a human-like Neu5Gc-deficient mouse model. Here we show that human anti-Neu5Gc antibodies can be affinity-purified in substantial amounts from clinically approved intravenous IgG (IVIG) and used at higher concentrations to suppress growth of the same Neu5Gc-expressing tumors. Hypothesizing that this polyclonal spectrum of human anti-Neu5Gc antibodies also includes potential cancer biomarkers, we then characterize them in cancer and noncancer patients' sera, using a novel sialoglycan microarray presenting multiple Neu5Gc-glycans and control Neu5Ac-glycans. Antibodies against Neu5Gcα2-6GalNAcα1-O-Ser/Thr (GcSTn) were found to be more prominent in patients with carcinomas than with other diseases. This unusual epitope arises from dietary Neu5Gc incorporation into the carcinoma marker Sialyl-Tn, and is the first example of such a novel mechanism for biomarker generation. Finally, human serum or purified antibodies rich in anti-GcSTn-reactivity kill GcSTn-expressing human tumors via complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. Such xeno-autoantibodies and xeno-autoantigens have potential for novel diagnostics, prognostics, and therapeutics in human carcinomas.
Conflict of interest statement
Figures



References
-
- Varki A, Kannagi R, Toole BP. Glycosylation Changes in Cancer. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. pp. 617–632. - PubMed
-
- Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–634. - PubMed
-
- Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175:228–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

