Intracellular fatty acids suppress β-adrenergic induction of PKA-targeted gene expression in white adipocytes
- PMID: 21505145
- PMCID: PMC3129835
- DOI: 10.1152/ajpendo.00039.2011
Intracellular fatty acids suppress β-adrenergic induction of PKA-targeted gene expression in white adipocytes
Abstract
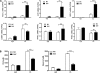
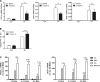
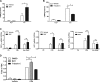
β-Adrenergic receptor (β-AR) activation elevates cAMP levels in fat cells and triggers both metabolic and transcriptional responses; however, the potential interactions between these pathways are poorly understood. This study investigated whether lipolysis affects β-AR-mediated gene expression in adipocytes. Acute β(3)-adrenergic receptor (β(3)-AR) stimulation with CL 316,243 (CL) increased expression of PKA-targeted genes PCG-1α, UCP1, and NOR-1 in mouse white fat. Limiting lipolysis via inhibition of hormone-sensitive lipase (HSL), a direct target of PKA, sharply potentiated CL induction of PCG-1α, UCP1, and NOR-1. CL also induced greater expression of PKA-targeted genes in white fat of HSL-null mice compared with wild-type littermates, further indicating that HSL activity limits PKA-mediated gene expression. Inhibiting HSL in 3T3-L1 adipocytes also potentiated the induction of PGC-1α, UCP1, and NOR-1 by β-AR activation, as did siRNA knockdown of adipose triglyceride lipase, the rate-limiting enzyme for lipolysis. Conversely, treatments that promote intracellular fatty acid accumulation suppressed induction of PGC-1α and UCP1 through β-AR stimulation. Analysis of β-adrenergic signaling indicated that excessive intracellular fatty acid production inhibits adenylyl cyclase activity and thereby reduces PKA signaling to the nucleus. Lastly, partially limiting lipolysis by inhibition of HSL increased the induction of oxidative gene expression and mitochondrial electron transport chain activity in white adipose tissue and facilitated fat loss in mice treated for 5 days with CL. Overall, our results demonstrate that fatty acids limit the upregulation of β-AR-responsive genes in white adipocytes and suggest that limiting lipolysis may be a novel means of enhancing β-AR signaling.
Figures
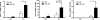
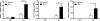
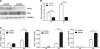
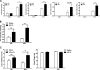

Similar articles
-
Reduced ATGL-mediated lipolysis attenuates β-adrenergic-induced AMPK signaling, but not the induction of PKA-targeted genes, in adipocytes and adipose tissue.Am J Physiol Cell Physiol. 2016 Aug 1;311(2):C269-76. doi: 10.1152/ajpcell.00126.2016. Epub 2016 Jun 29. Am J Physiol Cell Physiol. 2016. PMID: 27357546 Free PMC article.
-
Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue.Am J Physiol Endocrinol Metab. 2007 Nov;293(5):E1188-97. doi: 10.1152/ajpendo.00051.2007. Epub 2007 Aug 21. Am J Physiol Endocrinol Metab. 2007. PMID: 17711991
-
Novel Pharmacological Probes Reveal ABHD5 as a Locus of Lipolysis Control in White and Brown Adipocytes.J Pharmacol Exp Ther. 2017 Dec;363(3):367-376. doi: 10.1124/jpet.117.243253. Epub 2017 Sep 19. J Pharmacol Exp Ther. 2017. PMID: 28928121 Free PMC article.
-
The role of compartmentalized β-AR/cAMP signaling in the regulation of lipolysis in white and brown adipocytes.FEBS J. 2025 Jan;292(2):261-271. doi: 10.1111/febs.17157. Epub 2024 May 15. FEBS J. 2025. PMID: 38747241 Free PMC article. Review.
-
Lipolysis in adipocytes.Int J Biochem Cell Biol. 2010 May;42(5):555-9. doi: 10.1016/j.biocel.2009.12.009. Epub 2009 Dec 16. Int J Biochem Cell Biol. 2010. PMID: 20025992 Free PMC article. Review.
Cited by
-
Fatty acid signaling: the new function of intracellular lipases.Int J Mol Sci. 2015 Feb 10;16(2):3831-55. doi: 10.3390/ijms16023831. Int J Mol Sci. 2015. PMID: 25674855 Free PMC article. Review.
-
A FRET sensor for the real-time detection of long chain acyl-CoAs and synthetic ABHD5 ligands.Cell Rep Methods. 2023 Jan 25;3(2):100394. doi: 10.1016/j.crmeth.2023.100394. eCollection 2023 Feb 27. Cell Rep Methods. 2023. PMID: 36936069 Free PMC article.
-
Characterization of Eicosanoids Produced by Adipocyte Lipolysis: IMPLICATION OF CYCLOOXYGENASE-2 IN ADIPOSE INFLAMMATION.J Biol Chem. 2016 Jul 29;291(31):16001-10. doi: 10.1074/jbc.M116.725937. Epub 2016 May 31. J Biol Chem. 2016. PMID: 27246851 Free PMC article.
-
Carnosic Acid (CA) Induces a Brown Fat-like Phenotype, Increases Mitochondrial Biogenesis, and Activates AMPK in 3T3-L1 Adipocytes.Biomedicines. 2024 Jul 15;12(7):1569. doi: 10.3390/biomedicines12071569. Biomedicines. 2024. PMID: 39062141 Free PMC article.
-
Remodeling of lipid droplets during lipolysis and growth in adipocytes.J Biol Chem. 2012 Mar 30;287(14):11164-73. doi: 10.1074/jbc.M111.316794. Epub 2012 Feb 6. J Biol Chem. 2012. PMID: 22311986 Free PMC article.
References
-
- Ahmed W, Ziouzenkova O, Brown J, Devchand P, Francis S, Kadakia M, Kanda T, Orasanu G, Sharlach M, Zandbergen F, Plutzky J. PPARs and their metabolic modulation: new mechanisms for transcriptional regulation? J Intern Med 262: 184–198, 2007 - PubMed
-
- Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454: 470–477, 2008 - PubMed
-
- Bukowiecki LJ, Follea N, Lupien J, Paradis A. Metabolic relationships between lipolysis and respiration in rat brown adipocytes. The role of long chain fatty acids as regulators of mitochondrial respiration and feedback inhibitors of lipolysis. J Biol Chem 256: 12840–12848, 1981 - PubMed
-
- Burns TW, Langley PE, Terry BE, Robinson GA. The role of free fatty acids in the regulation of lipolysis by human adipose tissue cells. Metabolism 27: 1755–1762, 1978 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

