Exercise increases TBC1D1 phosphorylation in human skeletal muscle
- PMID: 21505148
- PMCID: PMC3129834
- DOI: 10.1152/ajpendo.00042.2011
Exercise increases TBC1D1 phosphorylation in human skeletal muscle
Abstract
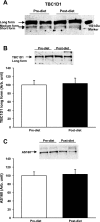
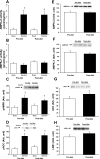
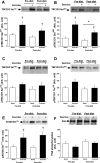
Exercise and weight loss are cornerstones in the treatment and prevention of type 2 diabetes, and both interventions function to increase insulin sensitivity and glucose uptake into skeletal muscle. Studies in rodents demonstrate that the underlying mechanism for glucose uptake in muscle involves site-specific phosphorylation of the Rab-GTPase-activating proteins AS160 (TBC1D4) and TBC1D1. Multiple kinases, including Akt and AMPK, phosphorylate TBC1D1 and AS160 on distinct residues, regulating their activity and allowing for GLUT4 translocation. In contrast to extensive rodent-based studies, the regulation of AS160 and TBC1D1 in human skeletal muscle is not well understood. In this study, we determined the effects of dietary intervention and a single bout of exercise on TBC1D1 and AS160 site-specific phosphorylation in human skeletal muscle. Ten obese (BMI 33.4 ± 2.4, M-value 4.3 ± 0.5) subjects were studied at baseline and after a 2-wk dietary intervention. Muscle biopsies were obtained from the subjects in the resting (basal) state and immediately following a 30-min exercise bout (70% Vo(2 max)). Muscle lysates were analyzed for AMPK activity and Akt phosphorylation and for TBC1D1 and AS160 phosphorylation on known or putative AMPK and Akt sites as follows: AS160 Ser(711) (AMPK), TBC1D1 Ser(231) (AMPK), TBC1D1 Ser(660) (AMPK), TBC1D1 Ser(700) (AMPK), and TBC1D1 Thr(590) (Akt). The diet intervention that consisted of a major shift in the macronutrient composition resulted in a 4.2 ± 0.4 kg weight loss (P < 0.001) and a significant increase in insulin sensitivity (M value 5.6 ± 0.6), but surprisingly, there was no effect on expression or phosphorylation of any of the muscle-signaling proteins. Exercise increased muscle AMPKα2 activity but did not increase Akt phosphorylation. Exercise increased phosphorylation on AS160 Ser(711), TBC1D1 Ser(231), and TBC1D1 Ser(660) but had no effect on TBC1D1 Ser(700). Exercise did not increase TBC1D1 Thr(590) phosphorylation or TBC1D1/AS160 PAS phosphorylation, consistent with the lack of Akt activation. These data demonstrate that a single bout of exercise regulates TBC1D1 and AS160 phosphorylation on multiple sites in human skeletal muscle.
Figures

Similar articles
-
Sustained AS160 and TBC1D1 phosphorylations in human skeletal muscle 30 min after a single bout of exercise.J Appl Physiol (1985). 2014 Aug 1;117(3):289-96. doi: 10.1152/japplphysiol.00044.2014. Epub 2014 May 29. J Appl Physiol (1985). 2014. PMID: 24876356 Free PMC article.
-
Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle.Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E242-51. doi: 10.1152/ajpendo.00194.2009. Epub 2009 May 12. Am J Physiol Endocrinol Metab. 2009. PMID: 19435856 Free PMC article.
-
Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation.J Biol Chem. 2008 Apr 4;283(14):9187-95. doi: 10.1074/jbc.M708934200. Epub 2008 Feb 7. J Biol Chem. 2008. PMID: 18258599 Free PMC article.
-
Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle.Diabetologia. 2015 Jan;58(1):19-30. doi: 10.1007/s00125-014-3395-5. Epub 2014 Oct 4. Diabetologia. 2015. PMID: 25280670 Free PMC article. Review.
-
Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport.Appl Physiol Nutr Metab. 2007 Jun;32(3):557-66. doi: 10.1139/H07-026. Appl Physiol Nutr Metab. 2007. PMID: 17510697 Review.
Cited by
-
α-Melanocyte stimulating hormone promotes muscle glucose uptake via melanocortin 5 receptors.Mol Metab. 2016 Aug 5;5(10):807-822. doi: 10.1016/j.molmet.2016.07.009. eCollection 2016 Oct. Mol Metab. 2016. PMID: 27688995 Free PMC article.
-
Skeletal muscle cell-specific differences in type 2 diabetes.Cell Mol Life Sci. 2022 Apr 23;79(5):256. doi: 10.1007/s00018-022-04265-7. Cell Mol Life Sci. 2022. PMID: 35460430 Free PMC article.
-
Multiomics analyses reveal dynamic bioenergetic pathways and functional remodeling of the heart during intermittent fasting.Elife. 2023 Sep 28;12:RP89214. doi: 10.7554/eLife.89214. Elife. 2023. PMID: 37769126 Free PMC article.
-
6-Mercaptopurine augments glucose transport activity in skeletal muscle cells in part via a mechanism dependent upon orphan nuclear receptor NR4A3.Am J Physiol Endocrinol Metab. 2013 Nov 1;305(9):E1081-92. doi: 10.1152/ajpendo.00169.2013. Epub 2013 Sep 10. Am J Physiol Endocrinol Metab. 2013. PMID: 24022864 Free PMC article.
-
Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle.Am J Physiol Endocrinol Metab. 2018 Nov 1;315(5):E859-E871. doi: 10.1152/ajpendo.00020.2018. Epub 2018 Aug 21. Am J Physiol Endocrinol Metab. 2018. PMID: 30130149 Free PMC article.
References
-
- No authors listed Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med 160: 898–904, 2000 - PubMed
-
- Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev 7: 49–58, 2006 - PubMed
-
- An D, Vichaiwong K, Toyoda T, Taylor EB, Yu H, Hirshman MF, Fujii N, Goodyear LJ. Contraction and insulin differentially regulate TBC1D1 phosphorylation and function in skeletal muscle (Abstract). Diabetes 58: A52, 2009.
-
- Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, MacKintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

