Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice
- PMID: 21505263
- PMCID: PMC3083767
- DOI: 10.1172/JCI43992
Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice
Abstract
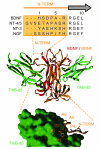
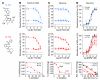
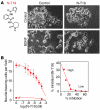
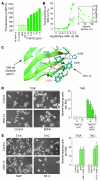
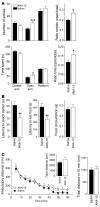
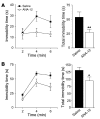
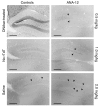
The neurotrophin brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin-related kinase B (TrkB) have emerged as key mediators in the pathophysiology of several mood disorders, including anxiety and depression. However, therapeutic compounds that interact with TrkB receptors have been difficult to develop. Using a combination of structure-based in silico screening and high-capacity functional assays in recombinant and neuronal cells, we identified a low-molecular weight TrkB ligand (ANA-12) that prevented activation of the receptor by BDNF with a high potency. ANA-12 showed direct and selective binding to TrkB and inhibited processes downstream of TrkB without altering TrkA and TrkC functions. KIRA-ELISA analysis demonstrated that systemic administration of ANA-12 to adult mice decreased TrkB activity in the brain without affecting neuronal survival. Mice administered ANA-12 demonstrated reduced anxiety- and depression-related behaviors on a variety of tests predictive of anxiolytic and antidepressant properties in humans. This study demonstrates that structure-based virtual screening strategy can be an efficient method for discovering potent TrkB-selective ligands that are active in vivo. We further propose that ANA-12 may be a valuable tool for studying BDNF/TrkB signaling and may constitute a lead compound for developing the next generation of therapeutic agents for the treatment of mood disorders.
Figures

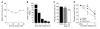
Comment in
-
Mood disorders: Small-molecule neurotrophin antagonist reduces anxiety.Nat Rev Drug Discov. 2011 Jun;10(6):415. doi: 10.1038/nrd3470. Nat Rev Drug Discov. 2011. PMID: 21629289 No abstract available.
References
-
- Yano H, Chao MV. Neurotrophin receptor structure and interactions. Pharm Acta Helv. 2000;74(2–3):253–260. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

