Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord
- PMID: 21505430
- PMCID: PMC3597081
- DOI: 10.1038/ncomms1276
Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord
Abstract
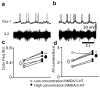
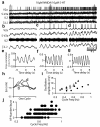
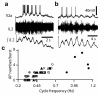
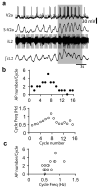
The principles governing the recruitment of interneurons during acceleration in vertebrate locomotion are unknown. In the mouse, the V2a spinal interneurons are dispensable for left-right coordination at low locomotor frequencies, but their function is essential for maintaining left-right coordination at high frequencies. Here we explore the mechanisms driving this frequency-dependent role using four methods to determine how V2a interneurons are recruited at different locomotor frequencies. We show that half of the V2a interneurons receive rhythmic locomotor synaptic drive, which increases with cycle frequency, recruiting more of the neurons to fire at higher frequencies. The other V2a interneurons do not receive locomotion-related synaptic drive and are not recruited into the locomotor network at any frequency. The increased role of V2a interneurons at higher locomotor frequencies arises from increased synaptic drive to recruit subthreshold oscillating V2a neurons, and not from recruitment of a second set of silent V2a interneurons.
Figures



References
-
- Kudo N, Yamada T. N-methyl-d,l-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci. Lett. 1987;75:43–48. - PubMed
-
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J. Neurosci. Methods. 1987;21:321–333. - PubMed
-
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J. Neurophysiol. 1997;77:247–259. - PubMed
-
- Kremer E, Lev-Tov A. Localization of the spinal network associated with the generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J. Neurophysiol. 1997;77:1155–1170. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

