N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis
- PMID: 21505478
- PMCID: PMC3170948
- DOI: 10.1038/jcbfm.2011.53
N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis
Abstract
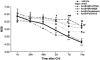
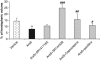
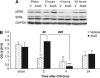
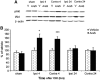
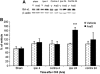
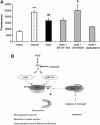
N-arachidonoyl-L-serine (AraS) is a brain component structurally related to the endocannabinoid family. We investigated the neuroprotective effects of AraS following closed head injury induced by weight drop onto the exposed fronto-parietal skull and the mechanisms involved. A single injection of AraS following injury led to a significant improvement in functional outcome, and to reduced edema and lesion volume compared with vehicle. Specific antagonists to CB2 receptors, transient receptor potential vanilloid 1 (TRPV1) or large conductance calcium-activated potassium (BK) channels reversed these effects. Specific binding assays did not indicate binding of AraS to the GPR55 cannabinoid receptor. N-arachidonoyl-L-serine blocked the attenuation in phosphorylated extracellular-signal-regulated kinase 1/2 (ERK) levels and led to an increase in pAkt in both the ipsilateral and contralateral cortices. Increased levels of the prosurvival factor Bcl-xL were evident 24 hours after injury in AraS-treated mice, followed by a 30% reduction in caspase-3 activity, measured 3 days after injury. Treatment with a CB2 antagonist, but not with a CB1 antagonist, reversed this effect. Our results suggest that administration of AraS leads to neuroprotection via ERK and Akt phosphorylation and induction of their downstream antiapoptotic pathways. These protective effects are related mostly to indirect signaling via the CB2R and TRPV1 channels but not through CB1 or GPR55 receptors.
Figures
Similar articles
-
N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury.J Cereb Blood Flow Metab. 2013 Aug;33(8):1242-50. doi: 10.1038/jcbfm.2013.75. Epub 2013 May 22. J Cereb Blood Flow Metab. 2013. PMID: 23695434 Free PMC article.
-
Palmitoyl Serine: An Endogenous Neuroprotective Endocannabinoid-Like Entity After Traumatic Brain Injury.J Neuroimmune Pharmacol. 2015 Jun;10(2):356-63. doi: 10.1007/s11481-015-9595-z. Epub 2015 Feb 27. J Neuroimmune Pharmacol. 2015. PMID: 25721934
-
N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties.Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2428-33. doi: 10.1073/pnas.0510676103. Epub 2006 Feb 7. Proc Natl Acad Sci U S A. 2006. PMID: 16467152 Free PMC article.
-
Are the endocannabinoid-like compounds N-acyl aminoacids neuroprotective after traumatic brain injury?J Basic Clin Physiol Pharmacol. 2016 May 1;27(3):209-16. doi: 10.1515/jbcpp-2015-0092. J Basic Clin Physiol Pharmacol. 2016. PMID: 26565551 Review.
-
The rise and fall of anandamide: processes that control synthesis, degradation, and storage.Mol Cell Biochem. 2021 Jul;476(7):2753-2775. doi: 10.1007/s11010-021-04121-5. Epub 2021 Mar 13. Mol Cell Biochem. 2021. PMID: 33713246 Review.
Cited by
-
Therapeutic hypothermia attenuates tissue damage and cytokine expression after traumatic brain injury by inhibiting necroptosis in the rat.Sci Rep. 2016 Apr 15;6:24547. doi: 10.1038/srep24547. Sci Rep. 2016. PMID: 27080932 Free PMC article.
-
N-Acyl Amino Acids: Metabolism, Molecular Targets, and Role in Biological Processes.Biomolecules. 2019 Dec 3;9(12):822. doi: 10.3390/biom9120822. Biomolecules. 2019. PMID: 31817019 Free PMC article. Review.
-
N-Oleoyl-glycine reduces nicotine reward and withdrawal in mice.Neuropharmacology. 2019 Apr;148:320-331. doi: 10.1016/j.neuropharm.2018.03.020. Epub 2018 Mar 19. Neuropharmacology. 2019. PMID: 29567093 Free PMC article.
-
Antihypertensive and neuroprotective effects of catestatin in spontaneously hypertensive rats: interaction with GABAergic transmission in amygdala and brainstem.Neuroscience. 2014 Jun 13;270:48-57. doi: 10.1016/j.neuroscience.2014.04.001. Epub 2014 Apr 13. Neuroscience. 2014. PMID: 24731867 Free PMC article.
-
Potential combinations of endocannabinoid/endocannabinoid-like compounds and antibiotics against methicillin-resistant Staphylococcus aureus.PLoS One. 2020 Apr 15;15(4):e0231583. doi: 10.1371/journal.pone.0231583. eCollection 2020. PLoS One. 2020. PMID: 32294120 Free PMC article.
References
-
- Barna I, Till I, Haller J. Blood, adipose tissue and brain levels of the cannabinoid ligands WIN-55,212 and SR-141716A after their intraperitoneal injection in mice: compound-specific and area-specific distribution within the brain. Eur Neuropsychopharmacol. 2009;19:533–541. - PubMed
-
- Beni-Adani L, Gozes I, Cohen Y, Assaf Y, Steingart RA, Brenneman DE, Eizenberg O, Trembolver V, Shohami E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther. 2001;296:57–63. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Chen XQ, Lau LT, Fung YW, Yu AC. Inactivation of Bad by site-specific phosphorylation: the checkpoint for ischemic astrocytes to initiate or resist apoptosis. J Neurosci Res. 2005;79:798–808. - PubMed
-
- Chen Y, McCarron RM, Ohara Y, Bembry J, Azzam N, Lenz FA, Shohami E, Mechoulam R, Spatz M. Human brain capillary endothelium: 2-arachidonoglycerol (endocannabinoid) interacts with endothelin-1. Circ Res. 2000;87:323–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

