Phase ii/iii study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21
- PMID: 21505599
- PMCID: PMC3070707
- DOI: 10.3747/co.v18i2.725
Phase ii/iii study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21
Abstract
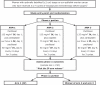
Three large randomized clinical trials have shown a survival benefit in women with stage iii epithelial ovarian cancer (eoc) who receive intraperitoneal (IP) chemotherapy after optimal primary debulking surgery. The most recent Gynecologic Oncology Group study, gog 172, showed an improvement in median overall survival of approximately 17 months. That result led to a U.S. National Cancer Institute (nci) clinical announcement recommending that IP chemotherapy be considered for this group of women with eoc. However, IP chemotherapy is associated with increased toxicity, and rates for completion of treatment are low (42% in gog 172). The optimal IP regimen and duration of treatment has yet to be defined. Women undergoing chemotherapy before optimal debulking surgery were not included in the studies or in the nci clinical announcement. The National Cancer Institute of Canada Clinical Trials Group has developed a protocol for a randomized phase ii/iii study which will examine whether IP platinum-taxane-based chemotherapy benefits women who have received neoadjuvant chemotherapy before optimal surgical debulking. To address whether the less systemically toxic carboplatin can be substituted for cisplatin IP, the first phase of the study will have 3 arms: 1 intravenous-only, and 2 IP-containing regimens. At the end of the first stage, and provided that IP therapy is feasible to administer in this patient population, one of the IP regimens, either IP carboplatin or IP cisplatin, will proceed into a phase iii comparison with the intravenous arm. This exciting new study has gathered international support.
Keywords: Intraperitoneal chemotherapy; epithelial ovarian cancer; ncic ctg ov.21; neoadjuvant chemotherapy.
Figures
References
-
- Dedrick RL, Myers CE, Bungay PM, DeVita VT., Jr Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. - PubMed
-
- Howell SB, Pfeifle CL, Wung WE, et al. Intraperitoneal cisplatin with systemic thiosulfate protection. Ann Intern Med. 1982;97:845–51. - PubMed
-
- Armstrong D, Bundy B, Wenzel L, et al. Phase iii randomized trial of intravenous cisplatin and paclitaxel versus an intensive regimen of intravenous paclitaxel, intraperitoneal cisplatin, and intraperitoneal paclitaxel in stage iii ovarian cancer: a Gynecologic Oncology Group study. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. - DOI - PubMed
LinkOut - more resources
Full Text Sources