Clusterin facilitates in vivo clearance of extracellular misfolded proteins
- PMID: 21505792
- PMCID: PMC11115182
- DOI: 10.1007/s00018-011-0684-8
Clusterin facilitates in vivo clearance of extracellular misfolded proteins
Abstract
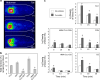
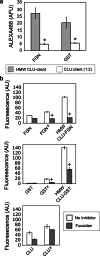
The extracellular deposition of misfolded proteins is a characteristic of many debilitating age-related disorders. However, little is known about the specific mechanisms that act to suppress this process in vivo. Clusterin (CLU) is an extracellular chaperone that forms stable and soluble complexes with misfolded client proteins. Here we explore the fate of complexes formed between CLU and misfolded proteins both in vitro and in a living organism. We show that proteins injected into rats are cleared more rapidly from circulation when complexed with CLU as a result of their more efficient localization to the liver and that this clearance is delayed by pre-injection with the scavenger receptor inhibitor fucoidan. The CLU-client complexes were found to bind preferentially, in a fucoidan-inhibitable manner, to human peripheral blood monocytes and isolated rat hepatocytes and in the latter cell type were internalized and targeted to lysosomes for degradation. The data suggest, therefore, that CLU plays a key role in an extracellular proteostasis system that recognizes, keeps soluble, and then rapidly mediates the disposal of misfolded proteins.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

