Association of endogenous anti-interferon-α autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus
- PMID: 21506093
- PMCID: PMC4028124
- DOI: 10.1002/art.30399
Association of endogenous anti-interferon-α autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus
Abstract
Objective: Numerous observations implicate interferon-α (IFNα) in the pathophysiology of systemic lupus erythematosus (SLE); however, the potential impact of endogenous anti-IFNα autoantibodies (AIAAs) on IFN-pathway and disease activity is unclear. The aim of this study was to characterize IFN-pathway activity and the serologic and clinical profiles of AIAA-positive patients with SLE.
Methods: Sera obtained from patients with SLE (n = 49), patients with rheumatoid arthritis (n = 25), and healthy control subjects (n = 25) were examined for the presence of AIAAs, using a biosensor immunoassay. Serum type I IFN bioactivity and the ability of AIAA-positive sera to neutralize IFNα activity were determined using U937 cells. Levels of IFN-regulated gene expression in peripheral blood were determined by microarray, and serum levels of BAFF, IFN-inducible chemokines, and other autoantibodies were measured using immunoassays.
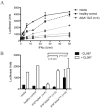
Results: AIAAs were detected in 27% of the serum samples from patients with SLE, using a biosensor immunoassay. Unsupervised hierarchical clustering analysis identified 2 subgroups of patients, IFN(low) and IFN(high) , that differed in the levels of serum type I IFN bioactivity, IFN-regulated gene expression, BAFF, anti-ribosomal P, and anti-chromatin autoantibodies, and in AIAA status. The majority of AIAA-positive patients had significantly lower levels of serum type I IFN bioactivity, reduced downstream IFN-pathway activity, and lower disease activity compared with the IFN(high) patients. AIAA-positive sera were able to effectively neutralize type I IFN activity in vitro.
Conclusion: Patients with SLE commonly harbor AIAAs. AIAA-positive patients have lower levels of serum type I IFN bioactivity and evidence for reduced downstream IFN-pathway and disease activity. AIAAs may influence the clinical course in SLE by blunting the effects produced by IFNα.
Copyright © 2011 by the American College of Rheumatology.
Figures


References
-
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
-
- Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus [review] Arthritis Rheum. 2006;54:408–20. - PubMed
-
- Hooks JJ, Jordan WG, Cuppos T, Montsopoulos TH, Fauci AS, Notkins AL. Multiple interferons in the circulation of patients with systemic lupus erythematosus and vasculitis. Arthritis Rheum. 1982;25:396–400. - PubMed
-
- Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science. 1982;216:429–31. - PubMed
-
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science. 2001;294:1540–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

