Knockdown of heme oxygenase-2 impairs corneal epithelial cell wound healing
- PMID: 21506105
- PMCID: PMC3078528
- DOI: 10.1002/jcp.22502
Knockdown of heme oxygenase-2 impairs corneal epithelial cell wound healing
Abstract
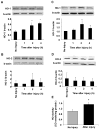
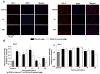
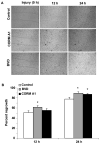
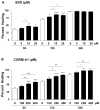
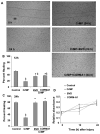
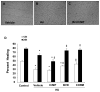
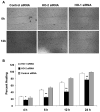
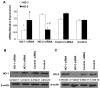
Heme oxygenase (HO) represents an intrinsic cytoprotective system based on its anti-oxidative and anti-inflammatory properties mediated via its products biliverdin/bilirubin and carbon monoxide (CO). We showed that deletion of HO-2 results in impaired corneal wound healing with associated chronic inflammatory complications. This study was undertaken to examine the role of HO activity and the contribution of HO-1 and HO-2 to corneal wound healing in an in vitro epithelial scratch injury model. A scratch wound model was established using human corneal epithelial (HCE) cells. These cells expressed both HO-1 and HO-2 proteins. Injury elicited a rapid and transient increase in HO-1 and HO activity; HO-2 expression was unchanged. Treatment with biliverdin or CORM-A1, a CO donor, accelerated wound closure by 10% at 24 h. Inhibition of HO activity impaired wound closure by more than 50%. However, addition of biliverdin or CORM-A1 reversed the effect of HO inhibition on wound healing. Moreover, knockdown of HO-2 expression, but not HO-1, significantly impaired wound healing. These results indicate that HO activity is required for corneal epithelial cell migration. Inhibition of HO activity impairs wound healing while amplification of its activity restores and accelerates healing. Importantly, HO-2, which is highly expressed in the corneal epithelium, appears to be critical for the wound healing process in the cornea. The mechanisms by which it contributes to cell migration in response to injury may reside in the cytoprotective properties of CO and biliverdin.
Copyright © 2010 Wiley-Liss, Inc.
Figures
Similar articles
-
Biliverdin Rescues the HO-2 Null Mouse Phenotype of Unresolved Chronic Inflammation Following Corneal Epithelial Injury.Invest Ophthalmol Vis Sci. 2011 May 17;52(6):3246-53. doi: 10.1167/iovs.10-6219. Invest Ophthalmol Vis Sci. 2011. PMID: 21345995 Free PMC article.
-
Targeted suppression of HO-2 gene expression impairs the innate anti-inflammatory and repair responses of the cornea to injury.Mol Vis. 2011 Apr 29;17:1144-52. Mol Vis. 2011. PMID: 21552471 Free PMC article.
-
Heme oxygenase-2 deletion impairs macrophage function: implication in wound healing.FASEB J. 2015 Jan;29(1):105-15. doi: 10.1096/fj.14-256503. Epub 2014 Oct 23. FASEB J. 2015. PMID: 25342128 Free PMC article.
-
Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers.Int J Mol Sci. 2022 Oct 10;23(19):12043. doi: 10.3390/ijms231912043. Int J Mol Sci. 2022. PMID: 36233341 Free PMC article. Review.
-
Heme oxygenase system and hypertension: a comprehensive insight.Curr Pharm Des. 2014;20(9):1354-69. doi: 10.2174/13816128113199990558. Curr Pharm Des. 2014. PMID: 23978093 Review.
Cited by
-
Pro-healing effects of bilirubin in open excision wound model in rats.Int Wound J. 2016 Jun;13(3):398-402. doi: 10.1111/iwj.12319. Epub 2014 Jun 20. Int Wound J. 2016. PMID: 24947136 Free PMC article.
-
Biliverdin Rescues the HO-2 Null Mouse Phenotype of Unresolved Chronic Inflammation Following Corneal Epithelial Injury.Invest Ophthalmol Vis Sci. 2011 May 17;52(6):3246-53. doi: 10.1167/iovs.10-6219. Invest Ophthalmol Vis Sci. 2011. PMID: 21345995 Free PMC article.
-
Dysregulated heme oxygenase-ferritin system in pterygium pathogenesis.Cornea. 2013 Sep;32(9):1276-82. doi: 10.1097/ICO.0b013e3182936915. Cornea. 2013. PMID: 23792437 Free PMC article.
-
The impact of heme oxygenase-2 on pharmacological research: A bibliometric analysis and beyond.Front Pharmacol. 2023 Apr 19;14:1156333. doi: 10.3389/fphar.2023.1156333. eCollection 2023. Front Pharmacol. 2023. PMID: 37153762 Free PMC article.
-
Age sensitizes the kidney to heme protein-induced acute kidney injury.Am J Physiol Renal Physiol. 2013 Feb 1;304(3):F317-25. doi: 10.1152/ajprenal.00606.2012. Epub 2012 Nov 28. Am J Physiol Renal Physiol. 2013. PMID: 23195679 Free PMC article.
References
-
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacological reviews. 2008;60(1):79–127. - PubMed
-
- Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9(12):2119–2137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

