Dearomatization strategies in the synthesis of complex natural products
- PMID: 21506209
- PMCID: PMC4136767
- DOI: 10.1002/anie.201006017
Dearomatization strategies in the synthesis of complex natural products
Abstract
Evolution in the field of the total synthesis of natural products has led to exciting developments over the last decade. Numerous chemoselective and enantioselective methodologies have emerged from total syntheses, resulting in efficient access to many important natural product targets. This Review highlights recent developments concerning dearomatization, a powerful strategy for the total synthesis of architecturally complex natural products wherein planar, aromatic scaffolds are converted to three-dimensional molecular architectures.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
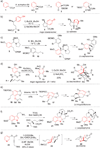


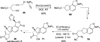

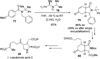
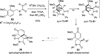

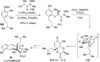


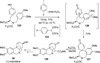
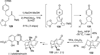
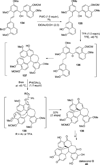

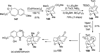
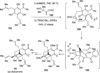












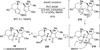
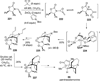
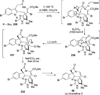

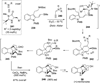
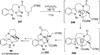
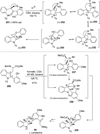


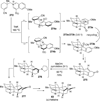


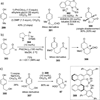
References
-
- Franklin JL. J. Am. Chem. Soc. 1950;72:4278–4280.
-
-
Myers AG, Siegel DR, Buzard DJ, Charest MG. Org. Lett. 2001;3:2923–2926. For a recent example, see: Mihovilovic MD, Leisch HG, Mereiter K. Tetrahedron Lett. 2004;45:7087–7090.
-
-
- Corey EJ, Girotra NN, Mathew CT. J. Am. Chem. Soc. 1969;91:1557–1559.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

