Ligand steric contours to understand the effects of N-heterocyclic carbene ligands on the reversal of regioselectivity in Ni-catalyzed reductive couplings of alkynes and aldehydes
- PMID: 21506540
- PMCID: PMC3128454
- DOI: 10.1021/ja202007s
Ligand steric contours to understand the effects of N-heterocyclic carbene ligands on the reversal of regioselectivity in Ni-catalyzed reductive couplings of alkynes and aldehydes
Abstract
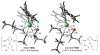
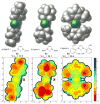
The regioselectivities of N-heterocyclic carbene (NHC) ligands in Ni-catalyzed alkyne-aldehyde reductive coupling reactions with silane reducing agents are investigated using density functional theory. Reversal of regioselectivity can be achieved by varying the steric bulkiness of the ligand. The steric influences of NHC ligands are highly anisotropic. Regioselectivity is primarily controlled by the steric hindrance at the region of the ligand close to the alkyne. Analysis of 2D contour maps of the NHC ligands indicates that the regioselectivities are directly affected by the shape and orientation of the N-substituents on the ligand.
© 2011 American Chemical Society
Figures


References
-
- Hoveyda AH, Evans DA, Fu GC. J Am Chem Soc. 1993;93:1307.
- Miller KM, Jamison TF. J Am Chem Soc. 2004;126:15342. - PubMed
- Bahadoor AB, Flyer A, Micalizio GC. J Am Chem Soc. 2005;127:3694. - PubMed
- Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Eur J Org Chem. 2010:391. - PMC - PubMed
- Liu P, Sirois LE, Cheong PHY, Yu ZX, Hartung IV, Rieck H, Wender PA, Houk KN. J Am Chem Soc. 2010;132:10127–10135. - PubMed
-
-
This strategy is exemplified by the acid-catalyzed hydration of olefins compared with hydroboration-oxidation protocols.
-
-
- Ohmura T, Oshima K, Taniguchi H, Suginome M. J Am Chem Soc. 2010;132:12194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

