Screening of protein-protein interaction modulators via sulfo-click kinetic target-guided synthesis
- PMID: 21506574
- PMCID: PMC3151735
- DOI: 10.1021/cb200085q
Screening of protein-protein interaction modulators via sulfo-click kinetic target-guided synthesis
Abstract
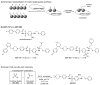
Kinetic target-guided synthesis (TGS) and in situ click chemistry are among unconventional discovery strategies having the potential to streamline the development of protein-protein interaction modulators (PPIMs). In kinetic TGS and in situ click chemistry, the target is directly involved in the assembly of its own potent, bidentate ligand from a pool of reactive fragments. Herein, we report the use and validation of kinetic TGS based on the sulfo-click reaction between thio acids and sulfonyl azides as a screening and synthesis platform for the identification of high-quality PPIMs. Starting from a randomly designed library consisting of 9 thio acids and 9 sulfonyl azides leading to 81 potential acylsulfonamides, the target protein, Bcl-X(L), selectively assembled four PPIMs, acylsulfonamides SZ4TA2, SZ7TA2, SZ9TA1, and SZ9TA5, which have been shown to modulate Bcl-X(L)/BH3 interactions. To further investigate the Bcl-X(L) templation effect, control experiments were carried out using two mutants of Bcl-X(L). In one mutant, phenylalanine Phe131 and aspartic acid Asp133, which are critical for the BH3 domain binding, were substituted by alanines, while arginine Arg139, a residue identified to play a crucial role in the binding of ABT-737, a BH3 mimetic, was replaced by an alanine in the other mutant. Incubation of these mutants with the reactive fragments and subsequent LC/MS-SIM analysis confirmed that these building block combinations yield the corresponding acylsulfonamides at the BH3 binding site, the actual "hot spot" of Bcl-X(L). These results validate kinetic TGS using the sulfo-click reaction as a valuable tool for the straightforward identification of high-quality PPIMs.
Figures





References
-
- Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature (London, United Kingdom) 2007;450:1001–1009. - PubMed
-
- Arkin M. Protein-protein interactions and cancer: small molecules going in for the kill. Current Opinion in Chemical Biology. 2005;9:317–324. - PubMed
-
- Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: Progressing towards the dream. Nature Reviews Drug Discovery. 2004;3:301–317. - PubMed
-
- Berg T. Modulation of protein-protein interactions with small organic molecules. Angewandte Chemie-International Edition. 2003;42:2462–2481. - PubMed
-
- Preissner R, Goede A, Frommel C. Dictionary of interfaces in proteins (DIP). Data bank of complementary molecular surface patches. Journal of Molecular Biology. 1998;280:535–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

