The expanding horizons of asparagine-linked glycosylation
- PMID: 21506607
- PMCID: PMC3101296
- DOI: 10.1021/bi200346n
The expanding horizons of asparagine-linked glycosylation
Abstract
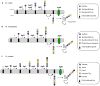
Asparagine-linked glycosylation involves the sequential assembly of an oligosaccharide onto a polyisoprenyl donor, followed by the en bloc transfer of the glycan to particular asparagine residues within acceptor proteins. These N-linked glycans play a critical role in a wide variety of biological processes, such as protein folding, cellular targeting and motility, and the immune response. In the past decade, research in the field of N-linked glycosylation has achieved major advances, including the discovery of new carbohydrate modifications, the biochemical characterization of the enzymes involved in glycan assembly, and the determination of the biological impact of these glycans on target proteins. It is now firmly established that this enzyme-catalyzed modification occurs in all three domains of life. However, despite similarities in the overall logic of N-linked glycoprotein biosynthesis among the three kingdoms, the structures of the appended glycans are markedly different and thus influence the functions of elaborated proteins in various ways. Though nearly all eukaryotes produce the same nascent tetradecasaccharide (Glc(3)Man(9)GlcNAc(2)), heterogeneity is introduced into this glycan structure after it is transferred to the protein through a complex series of glycosyl trimming and addition steps. In contrast, bacteria and archaea display diversity within their N-linked glycan structures through the use of unique monosaccharide building blocks during the assembly process. In this review, recent progress toward gaining a deeper biochemical understanding of this modification across all three kingdoms will be summarized. In addition, a brief overview of the role of N-linked glycosylation in viruses will also be presented.
Figures

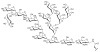

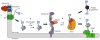



References
-
- Varki A, Cummings RD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology. 2. Cold Spring Harbor Laboratory Press; New York: 2009. - PubMed
-
- Walsh CT. Posttranslational modification of proteins: expanding nature's inventory. Roberts and Company Publishers; Greenwood Village, CO: 2006.
-
- Jensen PH, Kolarich D, Packer NH. Mucin-type O-glycosylation--putting the pieces together. FEBS J. 2010;277:81–94. - PubMed
-
- Hurtado-Guerrero R, Dorfmueller HC, van Aalten DMF. Molecular mechanisms of O-GlcNAcylation. Curr Opin Struct Biol. 2008;18:551–557. - PubMed
-
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

