Disulfide reduction in the endocytic pathway: immunological functions of gamma-interferon-inducible lysosomal thiol reductase
- PMID: 21506690
- PMCID: PMC3125571
- DOI: 10.1089/ars.2010.3684
Disulfide reduction in the endocytic pathway: immunological functions of gamma-interferon-inducible lysosomal thiol reductase
Abstract
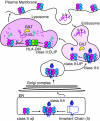
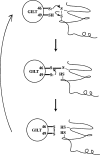
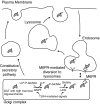
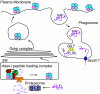
Gamma-interferon-inducible lysosomal thiol reductase (GILT) is constitutively expressed in most antigen presenting cells and is interferon γ inducible in other cell types via signal transducer and activator of transcription 1. Normally, N- and C-terminal propeptides are cleaved in the early endosome, and the mature protein resides in late endosomes and lysosomes. Correspondingly, GILT has maximal reductase activity at an acidic pH. Monocyte differentiation via Toll-like receptor 4 triggers secretion of a disulfide-linked dimer of the enzymatically active precursor, which may contribute to inflammation. GILT facilitates major histocompatibility complex (MHC) class II-restricted processing through reduction of protein disulfide bonds in the endocytic pathway and is hypothesized to expose buried epitopes for MHC class II binding. GILT can also facilitate the transfer of disulfide-containing antigens into the cytosol, enhancing their cross-presentation by MHC class I. A variety of antigens are strongly influenced by GILT-mediated reduction, including hen egg lysozyme, melanocyte differentiation antigens, and viral envelope glycoproteins. In addition, GILT is conserved among lower eukaryotes and likely has additional functions. For example, GILT expression increases the stability of superoxide dismutase 2 and decreases reactive oxygen species, which correlates with decreased cellular proliferation. It is also a critical host factor for infection with Listeria monocytogenes.
Figures

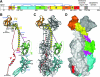
References
-
- Ackerman AL. Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004;5:678–684. - PubMed
-
- Amigorena S. Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22:109–117. - PubMed
-
- Barjaktarevic I. Rahman A. Radoja S. Bogunovic B. Vollmer A. Vukmanovic S. Maric M. Inhibitory role of IFN-{gamma}-inducible lysosomal thiol reductase in T cell activation. J Immunol. 2006;177:4369–4375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

