A common MUC5B promoter polymorphism and pulmonary fibrosis
- PMID: 21506741
- PMCID: PMC3379886
- DOI: 10.1056/NEJMoa1013660
A common MUC5B promoter polymorphism and pulmonary fibrosis
Abstract
Background: The mutations that have been implicated in pulmonary fibrosis account for only a small proportion of the population risk.
Methods: Using a genomewide linkage scan, we detected linkage between idiopathic interstitial pneumonia and a 3.4-Mb region of chromosome 11p15 in 82 families. We then evaluated genetic variation in this region in gel-forming mucin genes expressed in the lung among 83 subjects with familial interstitial pneumonia, 492 subjects with idiopathic pulmonary fibrosis, and 322 controls. MUC5B expression was assessed in lung tissue.
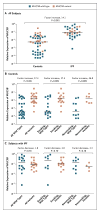
Results: Linkage and fine mapping were used to identify a region of interest on the p-terminus of chromosome 11 that included gel-forming mucin genes. The minor-allele of the single-nucleotide polymorphism (SNP) rs35705950, located 3 kb upstream of the MUC5B transcription start site, was present at a frequency of 34% among subjects with familial interstitial pneumonia, 38% among subjects with idiopathic pulmonary fibrosis, and 9% among controls (allelic association with familial interstitial pneumonia, P=1.2×10(-15); allelic association with idiopathic pulmonary fibrosis, P=2.5×10(-37)). The odds ratios for disease among subjects who were heterozygous and those who were homozygous for the minor allele of this SNP were 6.8 (95% confidence interval [CI], 3.9 to 12.0) and 20.8 (95% CI, 3.8 to 113.7), respectively, for familial interstitial pneumonia and 9.0 (95% CI, 6.2 to 13.1) and 21.8 (95% CI, 5.1 to 93.5), respectively, for idiopathic pulmonary fibrosis. MUC5B expression in the lung was 14.1 times as high in subjects who had idiopathic pulmonary fibrosis as in those who did not (P<0.001). The variant allele of rs35705950 was associated with up-regulation in MUC5B expression in the lung in unaffected subjects (expression was 37.4 times as high as in unaffected subjects homozygous for the wild-type allele, P<0.001). MUC5B protein was expressed in lesions of idiopathic pulmonary fibrosis.
Conclusions: A common polymorphism in the promoter of MUC5B is associated with familial interstitial pneumonia and idiopathic pulmonary fibrosis. Our findings suggest that dysregulated MUC5B expression in the lung may be involved in the pathogenesis of pulmonary fibrosis. (Funded by the National Heart, Lung, and Blood Institute and others.).
Figures



Comment in
-
Idiopathic pulmonary fibrosis--a sticky business.N Engl J Med. 2011 Apr 21;364(16):1560-1. doi: 10.1056/NEJMe1014191. N Engl J Med. 2011. PMID: 21506745 No abstract available.
-
MUC5B promoter polymorphism and pulmonary fibrosis.N Engl J Med. 2011 Jul 14;365(2):178; author reply 178-9. doi: 10.1056/NEJMc1105707. N Engl J Med. 2011. PMID: 21751916 No abstract available.
-
MUC5B promoter polymorphism and pulmonary fibrosis.N Engl J Med. 2011 Jul 14;365(2):178; author reply 178-9. doi: 10.1056/NEJMc1105707. N Engl J Med. 2011. PMID: 21751917 No abstract available.
-
MUC5B and pulmonary fibrosis, omalizumab for severe allergic asthma, and interstitial lung abnormalities in smokers with emphysema.Am J Respir Crit Care Med. 2012 May 1;185(9):1021-2. doi: 10.1164/rccm.201110-1846RR. Am J Respir Crit Care Med. 2012. PMID: 22550211 No abstract available.
References
-
- Raghu G, Hert R. Interstitial lung diseases: genetic predisposition and inherited interstitial lung diseases. Sem Respir Med. 1993;14:323–32.
-
- Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–9. - PubMed
-
- Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL036982/HL/NHLBI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R37-HL36982/HL/NHLBI NIH HHS/United States
- Z01 ES101947/ImNIH/Intramural NIH HHS/United States
- R01-HL095393/HL/NHLBI NIH HHS/United States
- P01-HL092870/HL/NHLBI NIH HHS/United States
- R01-HL097163/HL/NHLBI NIH HHS/United States
- U01-HL067467/HL/NHLBI NIH HHS/United States
- RC2-HL101715/HL/NHLBI NIH HHS/United States
- RC2 HL101715/HL/NHLBI NIH HHS/United States
- P01 HL092870/HL/NHLBI NIH HHS/United States
- U01 HL067467/HL/NHLBI NIH HHS/United States
- P30-CA016672/CA/NCI NIH HHS/United States
- R01 HL097163/HL/NHLBI NIH HHS/United States
- R01 HL095393/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
