Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study
- PMID: 21507477
- PMCID: PMC3085933
- DOI: 10.1016/S0140-6736(11)60438-8
Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study
Abstract
Background: The Xpert MTB/RIF test (Cepheid, Sunnyvale, CA, USA) can detect tuberculosis and its multidrug-resistant form with very high sensitivity and specificity in controlled studies, but no performance data exist from district and subdistrict health facilities in tuberculosis-endemic countries. We aimed to assess operational feasibility, accuracy, and effectiveness of implementation in such settings.
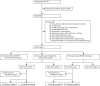
Methods: We assessed adults (≥18 years) with suspected tuberculosis or multidrug-resistant tuberculosis consecutively presenting with cough lasting at least 2 weeks to urban health centres in South Africa, Peru, and India, drug-resistance screening facilities in Azerbaijan and the Philippines, and an emergency room in Uganda. Patients were excluded from the main analyses if their second sputum sample was collected more than 1 week after the first sample, or if no valid reference standard or MTB/RIF test was available. We compared one-off direct MTB/RIF testing in nine microscopy laboratories adjacent to study sites with 2-3 sputum smears and 1-3 cultures, dependent on site, and drug-susceptibility testing. We assessed indicators of robustness including indeterminate rate and between-site performance, and compared time to detection, reporting, and treatment, and patient dropouts for the techniques used.
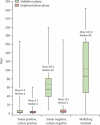
Findings: We enrolled 6648 participants between Aug 11, 2009, and June 26, 2010. One-off MTB/RIF testing detected 933 (90·3%) of 1033 culture-confirmed cases of tuberculosis, compared with 699 (67·1%) of 1041 for microscopy. MTB/RIF test sensitivity was 76·9% in smear-negative, culture-positive patients (296 of 385 samples), and 99·0% specific (2846 of 2876 non-tuberculosis samples). MTB/RIF test sensitivity for rifampicin resistance was 94·4% (236 of 250) and specificity was 98·3% (796 of 810). Unlike microscopy, MTB/RIF test sensitivity was not significantly lower in patients with HIV co-infection. Median time to detection of tuberculosis for the MTB/RIF test was 0 days (IQR 0-1), compared with 1 day (0-1) for microscopy, 30 days (23-43) for solid culture, and 16 days (13-21) for liquid culture. Median time to detection of resistance was 20 days (10-26) for line-probe assay and 106 days (30-124) for conventional drug-susceptibility testing. Use of the MTB/RIF test reduced median time to treatment for smear-negative tuberculosis from 56 days (39-81) to 5 days (2-8). The indeterminate rate of MTB/RIF testing was 2·4% (126 of 5321 samples) compared with 4·6% (441 of 9690) for cultures.
Interpretation: The MTB/RIF test can effectively be used in low-resource settings to simplify patients' access to early and accurate diagnosis, thereby potentially decreasing morbidity associated with diagnostic delay, dropout and mistreatment.
Funding: Foundation for Innovative New Diagnostics, Bill & Melinda Gates Foundation, European and Developing Countries Clinical Trials Partnership (TA2007.40200.009), Wellcome Trust (085251/B/08/Z), and UK Department for International Development.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Improving tuberculosis diagnostics and treatment.Lancet. 2011 Apr 30;377(9776):1467-8. doi: 10.1016/S0140-6736(11)60513-8. Epub 2011 Apr 18. Lancet. 2011. PMID: 21507476 No abstract available.
-
Xpert MTB/RIF test for tuberculosis.Lancet. 2011 Aug 6;378(9790):481; author reply 482-3. doi: 10.1016/S0140-6736(11)61242-7. Lancet. 2011. PMID: 21821176 No abstract available.
-
Xpert MTB/RIF test for tuberculosis.Lancet. 2011 Aug 6;378(9790):481-2; author reply 482-3. doi: 10.1016/S0140-6736(11)61243-9. Lancet. 2011. PMID: 21821177 No abstract available.
-
Xpert MTB/RIF test for tuberculosis.Lancet. 2011 Aug 6;378(9790):482; author reply 482-3. doi: 10.1016/S0140-6736(11)61244-0. Lancet. 2011. PMID: 21821179 No abstract available.
-
A single Xpert MTB/RIF test of sputum for diagnosis of tuberculosis and multidrug resistance shows high sensitivity and specificity and reduces diagnosis and treatment delays.Evid Based Med. 2011 Dec;16(6):174-5. doi: 10.1136/ebm.2011.100207. Epub 2011 Oct 11. Evid Based Med. 2011. PMID: 21990187 No abstract available.
References
-
- Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Kallenius G, Lindquist L. Sputum concentration improves diagnosis of tuberculosis in a setting with a high prevalence of HIV. Trans R Soc Trop Med Hyg. 2000;94:677–680. - PubMed
-
- Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196:S15–S27. - PubMed
-
- Steingart KR, Ramsay A, Pai M. Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev Anti Infect Ther. 2007;5:327–331. - PubMed
-
- World Health Organization Global tuberculosis control. 2010. http://whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf (accessed March 18, 2011).
-
- Behr MA, Warren SA, Salamon H. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

