Effects of protein dose and delivery system on BMP-mediated bone regeneration
- PMID: 21507479
- PMCID: PMC3129848
- DOI: 10.1016/j.biomaterials.2011.03.063
Effects of protein dose and delivery system on BMP-mediated bone regeneration
Abstract
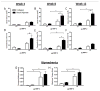
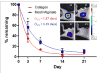
Delivery of recombinant proteins is a proven therapeutic strategy to promote endogenous repair mechanisms and tissue regeneration. Bone morphogenetic protein-2 (rhBMP-2) has been used to promote spinal fusion and repair of challenging bone defects; however, the current clinically-used carrier, absorbable collagen sponge, requires high doses and has been associated with adverse complications. We evaluated the hypothesis that the relationship between protein dose and regenerative efficacy depends on delivery system. First, we determined the dose-response relationship for rhBMP-2 delivered to 8-mm rat bone defects in a hybrid nanofiber mesh/alginate delivery system at six doses ranging from 0 to 5 μg. Next, we directly compared the hybrid delivery system to the collagen sponge at 0.1 and 1.0 μg. Finally, we compared the in vivo protein release properties of the two delivery methods. In the hybrid delivery system, bone volume, connectivity and mechanical properties increased in a dose-dependent manner to rhBMP-2. Consistent bridging of the defect was observed for doses of 1.0 μg and greater. Compared to collagen sponge delivery at the same 1.0 μg dose, the hybrid system yielded greater connectivity by week 4 and 2.5-fold greater bone volume by week 12. These differences may be explained by the significantly greater protein retention in the hybrid system compared to collagen sponge. This study demonstrates a clear dose-dependent effect of rhBMP-2 delivered using a hybrid nanofiber mesh/alginate delivery system. Furthermore, the effective dose was found to vary with delivery system, demonstrating the importance of biomaterial carrier properties in the delivery of recombinant proteins.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





References
-
- Sasso RC, LeHuec JC, Shaffrey C. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77–81. - PubMed
-
- Rihn JA, Kirkpatrick K, Albert TJ. Graft options in posterolateral and posterior interbody lumbar fusion. Spine (Phila Pa 1976) 2010;3517:1629–1639. - PubMed
-
- Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;122:212–218. - PubMed
-
- Berrey BH, Jr, Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;726:825–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

