Supply and demand for endocannabinoids
- PMID: 21507493
- PMCID: PMC3106144
- DOI: 10.1016/j.tins.2011.03.003
Supply and demand for endocannabinoids
Abstract
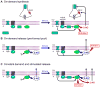
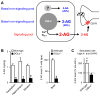
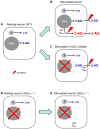
The endocannabinoid system consists of G-protein-coupled cannabinoid receptors that can be activated by cannabis-derived drugs and small lipids termed endocannabinoids (eCBs) plus associated biochemical machinery (precursors, synthetic and degradative enzymes, transporters). The eCB system in the brain primarily influences neuronal synaptic communication, and affects biological functions - including eating, anxiety, learning and memory, growth and development - via an array of actions throughout the nervous system. Although many aspects of synaptic regulation by eCBs are becoming clear, details of the subcellular organization and regulation of the eCB system are less well understood. This review focuses on recent investigations that illuminate fundamental issues of eCB storage, release, and functional roles.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647.
-
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. - PubMed
-
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. - PubMed
-
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

