Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy
- PMID: 21507924
- PMCID: PMC3079399
- DOI: 10.1093/cid/cir131
Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy
Abstract
Background: Adequate exposure to antiretrovirals is important to maintain durable responses, but methods to assess exposure (eg, querying adherence and single plasma drug level measurements) are limited. Hair concentrations of antiretrovirals can integrate adherence and pharmacokinetics into a single assay.
Methods: Small hair samples were collected from participants in the Women's Interagency HIV Study (WIHS), a large cohort of human immunodeficiency virus (HIV)-infected (and at-risk noninfected) women. From 2003 through 2008, we analyzed atazanavir hair concentrations longitudinally for women reporting receipt of atazanavir-based therapy. Multivariate random effects logistic regression models for repeated measures were used to estimate the association of hair drug levels with the primary outcome of virologic suppression (HIV RNA level, <80 copies/mL).
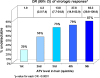
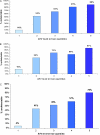
Results: 424 WIHS participants (51% African-American, 31% Hispanic) contributed 1443 person-visits to the analysis. After adjusting for age, race, treatment experience, pretreatment viral load, CD4 count and AIDS status, and self-reported adherence, hair levels were the strongest predictor of suppression. Categorized hair antiretroviral levels revealed a monotonic relationship to suppression; women with atazanavir levels in the highest quintile had odds ratios (ORs) of 59.8 (95% confidence ratio, 29.0-123.2) for virologic suppression. Hair atazanavir concentrations were even more strongly associated with resuppression of viral loads in subgroups in which there had been previous lapses in adherence (OR, 210.2 [95% CI, 46.0-961.1]), low hair levels (OR, 132.8 [95% CI, 26.5-666.0]), or detectable viremia (OR, 400.7 [95% CI, 52.3-3069.7]).
Conclusions: Antiretroviral hair levels surpassed any other predictor of virologic outcomes to HIV treatment in a large cohort. Low antiretroviral exposure in hair may trigger interventions prior to failure or herald virologic failure in settings where measurement of viral loads is unavailable. Monitoring hair antiretroviral concentrations may be useful for prolonging regimen durability.
Figures

Similar articles
-
Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy after sustained virologic suppression.JAMA. 2006 Aug 16;296(7):806-14. doi: 10.1001/jama.296.7.806. JAMA. 2006. PMID: 16905786 Clinical Trial.
-
Protease inhibitor levels in hair strongly predict virologic response to treatment.AIDS. 2009 Feb 20;23(4):471-8. doi: 10.1097/QAD.0b013e328325a4a9. AIDS. 2009. PMID: 19165084 Free PMC article.
-
Nevirapine Concentration in Hair Samples Is a Strong Predictor of Virologic Suppression in a Prospective Cohort of HIV-Infected Patients.PLoS One. 2015 Jun 8;10(6):e0129100. doi: 10.1371/journal.pone.0129100. eCollection 2015. PLoS One. 2015. PMID: 26053176 Free PMC article.
-
Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir.Clin Pharmacokinet. 2005;44(10):1035-50. doi: 10.2165/00003088-200544100-00003. Clin Pharmacokinet. 2005. PMID: 16176117 Review.
-
Atazanavir: in pediatric patients with HIV-1 infection.Paediatr Drugs. 2012 Apr 1;14(2):131-41. doi: 10.2165/11208550-000000000-00000. Paediatr Drugs. 2012. PMID: 22292486 Review.
Cited by
-
Tenofovir and emtricitabine concentrations in hair are comparable between individuals on tenofovir disoproxil fumarate versus tenofovir alafenamide-based ART.Drug Test Anal. 2021 Jul;13(7):1354-1370. doi: 10.1002/dta.3033. Epub 2021 Apr 13. Drug Test Anal. 2021. PMID: 33742745 Free PMC article.
-
Therapeutic drug monitoring of corticosteroids/β2-agonists in the hair of patients with asthma: an open-label feasibility study.Front Pharmacol. 2024 Jan 10;14:1339835. doi: 10.3389/fphar.2023.1339835. eCollection 2023. Front Pharmacol. 2024. PMID: 38269282 Free PMC article.
-
Measuring Adherence to Antiretroviral Therapy via Hair Concentrations in India.J Acquir Immune Defic Syndr. 2019 Jun 1;81(2):202-206. doi: 10.1097/QAI.0000000000001993. J Acquir Immune Defic Syndr. 2019. PMID: 30865182 Free PMC article.
-
Adherence to Antiretroviral Therapy and Pre-exposure Prophylaxis: TARGETing the Ideal Measure.Clin Infect Dis. 2020 May 6;70(10):2152-2154. doi: 10.1093/cid/ciz651. Clin Infect Dis. 2020. PMID: 31314075 Free PMC article. No abstract available.
-
Antiretroviral bioanalysis methods of tissues and body biofluids.Bioanalysis. 2013 Feb;5(3):351-68. doi: 10.4155/bio.12.319. Bioanalysis. 2013. PMID: 23394701 Free PMC article. Review.
References
-
- Bisson GP, Gross R, Strom JB, et al. Diagnostic accuracy of CD4 cell count increase for virologic response after initiating highly active antiretroviral therapy. AIDS. 2006;20:1613–9. - PubMed
-
- Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization's global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(suppl 2):1–13. - PubMed
-
- Bertagnolio S, Kelley K, Hassani AS, et al. Boston, MA: 2011. Surveillance of transmitted and acquired HIV drug resistance using WHO surveys in resource-limited settings. 18th Conference on Retroviruses and Opportunistic Infections.
-
- Gandhi M, Greenblatt RM. Hair it is: the long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137:696–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UO1-HD-32632/HD/NICHD NIH HHS/United States
- UO1-AI-34994/AI/NIAID NIH HHS/United States
- UO1-AI-34989/AI/NIAID NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- UO1-AI-35004/AI/NIAID NIH HHS/United States
- UO1-AI-34993/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- R01-AI-65233/AI/NIAID NIH HHS/United States
- UO1-AI-42590/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- UO1-AI-31834/AI/NIAID NIH HHS/United States
- R01 AI065233/AI/NIAID NIH HHS/United States
- K23 A1067065/PHS HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

